Ultrafast decomposition of refractory organics by electric field enhanced permanganate/peroxymonosulfate process
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
A novel water treatment process called electrolysis-enhanced permanganate/peroxymonosulfate (E-PM-PMS) was investigated. The process demonstrated rapid degradation of persistent organic pollutants, such as carbamazepine (CBZ), diclofenac (DCF), and tetracycline (TC). For the degradation of ibuprofen (IBP), the E-PM-PMS process showed the highest mineralization ratio (55.76 %) and the lowest energy consumption (8.930 kWh m−3) within 120 min, compared with the PM-PMS (25.93 %, 61.814 kWh m−3) and E-PMS (31.07 %, 67.903 kWh m−3) processes. Mechanism studies indicated that the introduction of an electric field could promote the Mn(VII)/Mn(VI) cycle to form more permanganate (VI) complexes, resulting in the generation of more active species. Additionally, the MnO2 produced in large amounts during the reaction could activate PMS to generate active species through the Mn(III)/Mn(IV) cycle, while also being activated by these active species to form Mn(V) and Mn(VI). Both the electric field and PMS reacting with PM led to the formation of various RMnS species (Mn(VI)aq, Mn(V)aq, Mn(IV)s, and Mn(III)aq). Through radical scavenging, electron paramagnetic resonance (EPR) experiments, it was proved that the degradation of IBP was mainly driven by reactive radicals, which are (72.24 %) and •OH (12.38 %). Twelve intermediate products were detected in total, and four possible degradation pathways for ibuprofen were identified. Within a certain range, increasing PMS concentration, PM dosage, current density, NO3− concentration, and decreasing the pH improved the efficiency of IBP elimination in the E-PM-PMS process, while Cl−, HCO3−, PO43−, and humic acid hindered it. Overall, the E-PM-PMS process shows promise as an economical, eco-friendly, and efficient water treatment technique
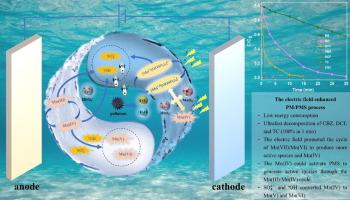
电场强化高锰酸盐/过氧单硫酸盐法超快分解难降解有机物
研究了电解强化高锰酸盐/过氧单硫酸盐(E-PM-PMS)水处理新工艺。该过程证明了持久性有机污染物的快速降解,如卡马西平(CBZ)、双氯芬酸(DCF)和四环素(TC)。对于ibuprofen (IBP)的降解,E-PM-PMS工艺在120 min内矿化率最高(55.76 %),能耗最低(8.930 kWh m−3),而PM-PMS工艺(25.93 %,61.814 kWh m−3)和E-PMS工艺(31.07 %,67.903 kWh m−3)。机理研究表明,电场的引入可以促进Mn(VII)/Mn(VI)循环形成更多的高锰酸盐(VI)配合物,从而产生更多的活性物质。此外,在反应过程中大量产生的MnO2可以激活PMS通过Mn(III)/Mn(IV)循环生成活性物质,同时也被这些活性物质激活形成Mn(V)和Mn(VI)。电场和PMS与PM的反应均可生成各种RMnS (Mn(VI)aq、Mn(V)aq、Mn(IV)s和Mn(III)aq)。通过自由基清除和电子顺磁共振(EPR)实验证明,IBP的降解主要由活性自由基驱动,即SO4∙-SO4∙-(72.24 %)和•OH(12.38 %)。共检测到12个中间产物,确定了4种可能的布洛芬降解途径。在一定范围内,增加PMS浓度、PM投加量、电流密度、NO3−浓度和降低pH均能提高E-PM-PMS过程中IBP的消除效率,而Cl−、HCO3−、PO43−和腐植酸则会阻碍IBP的消除。总的来说,E-PM-PMS工艺作为一种经济、环保、高效的水处理技术显示出前景
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


