Magnesium–Phenolic Nanoeditor Refining Gliomatous T Cells for Metalloimmunotherapy
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
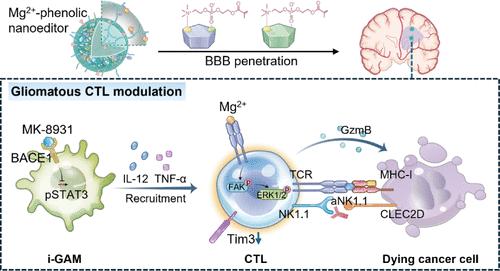
More than the sparse infiltration in glioblastoma, cytotoxic T lymphocytes (CTLs) also function inefficiently and overexpress the inhibitory markers, especially the identified NK cell receptor (NK1.1). However, most studies solely focus on how to augment tumor-infiltrating CTLs and overlook their killing maintenance. Metalloimmunotherapy has been proven to improve the functionalities of CTLs, but it has barely adapted to glioblastoma due to the severe limitations of safe delivery and the brain’s physiological properties. Herein, we synthesized an amphipathic polyethylene glycol (PEG) polymer (designated as MPP) modified with the choline analogue 2-methacryloyloxyethyl phosphorylcholine (MPC) and polyphenol moieties to customize a nanoeditor (Mg2+@MK-8931@MPP) by coordinating Mg2+ and entrapping the hydrophobic BACE1 inhibitor MK-8931, then precisely redressing the gliomatous CTL sparsity and cytotoxic dysfunction. Upon MPC-assisted local accumulation in glioblastoma, Mg2+@MK-8931@MPP nanoeditors release MK-8931 to repolarize M2-like macrophages, facilitating CTL infiltration quantitatively. The cenogenetic immune adjuvant Mg2+ ulteriorly fortifies the T-cell receptor downstream signals to enhance the functionality of the ingoing CTLs in quality, leading to the secretion of high-level antitumor cytokines and cytotoxic proteins. Further blocking the inhibitory NK1.1 on CTLs by anti-NK1.1 antibodies can extend their cytolytic endgame. Studies on T-cell-deficient and wild-type mouse models support the immunomodulating feasibility of Mg2+@MK-8931@MPP. This gliomatous CTL-tailored strategy concurrently broadens metalloimmunotherapy to glioblastoma treatment and highlights the necessity of enforcing gliomatous CTLs’ functionality.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


