Robust, Scalable Microfluidic Manufacturing of RNA–Lipid Nanoparticles Using Immobilized Antifouling Lubricant Coating
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
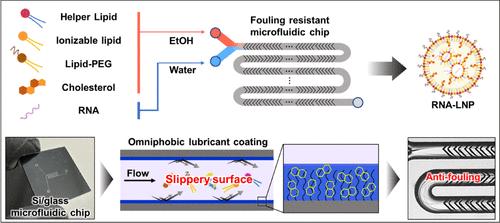
Despite the numerous advantages demonstrated by microfluidic mixing for RNA-loaded lipid nanoparticle (RNA-LNP) production over bulk methods, such as precise size control, homogeneous distributions, higher encapsulation efficiencies, and improved reproducibility, their translation from research to commercial manufacturing remains elusive. A persistent challenge hindering the adoption of microfluidics for LNP production is the fouling of device surfaces during prolonged operation, which significantly diminishes performance and reliability. The complexity of LNP constituents, including lipids, cholesterol, RNA, and solvent mixtures, makes it difficult to find a single coating that can prevent fouling. To address this challenge, we propose using an immobilized liquid lubricant layer of perfluorodecalin (PFD) to create an antifouling surface that can repel the multiple LNP constituents. We apply this technology to a staggered herringbone microfluidic (SHM) mixing chip and achieve >3 h of stable operation, a >15× increase relative to gold standard approaches. We also demonstrate the compatibility of this approach with a parallelized microfluidic platform that incorporates 256 SHM mixers, with which we demonstrate scale up, stable production at L/h production rates suitable for commercial scale applications. We verify that the LNPs produced on our chip match both the physiochemical properties and performance for both in vitro and in vivo mRNA delivery as those made on chips without the coating. By suppressing surface fouling with an immobilized liquid lubricant layer, this technology not only enhances RNA-LNP production but also promises to transform the microfluidic manufacturing of diverse materials, ensuring more reliable and robust processes.
固定化防污润滑剂涂层制备rna -脂质纳米颗粒的鲁棒、可扩展微流控技术
尽管与散装方法相比,微流控混合rna负载脂质纳米颗粒(RNA-LNP)的生产具有许多优势,如精确的尺寸控制、均匀的分布、更高的封装效率和更好的可重复性,但它们从研究到商业生产的转化仍然难以捉摸。长期以来,阻碍微流体技术应用于LNP生产的一个挑战是,在长时间运行过程中,设备表面的结垢会显著降低性能和可靠性。LNP成分的复杂性,包括脂质、胆固醇、RNA和溶剂混合物,使得很难找到一种可以防止污染的单一涂层。为了解决这一挑战,我们建议使用全氟十烷(PFD)的固定化液体润滑层来创建一个防污表面,可以抵抗多种LNP成分。我们将该技术应用于交错人字形微流控(SHM)混合芯片,实现了3小时的稳定运行,比金标准方法提高了15倍。我们还演示了这种方法与包含256个SHM混合器的并行微流控平台的兼容性,通过该平台,我们展示了适合商业规模应用的L/h生产速率的规模化、稳定生产。我们验证了在我们的芯片上生产的LNPs在体外和体内mRNA传递的物理化学性质和性能与在没有涂层的芯片上生产的LNPs相匹配。通过固定化液体润滑层抑制表面污垢,该技术不仅提高了RNA-LNP的生产,而且有望改变各种材料的微流控制造,确保更可靠和强大的工艺。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


