Competing Photocleavage on Boron and at the meso-Position in BODIPY Photocages
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
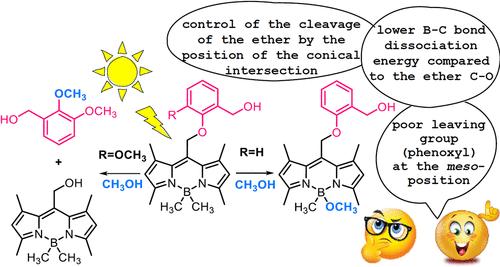
BODIPY photocages (photocleavable protective groups) have stirred interest because they can release biologically active cargo upon visible light excitation. We conducted combined theoretical and experimental investigations on selected BODIPY photocages to elucidate the mechanism of the competing photocleavage at the boron and meso-position. Based on the computations, the former reaction involves elongation of the B–C bond, yielding a tight borenium cation and methyl anion. These ions are intercepted by CH3OH, enabling an efficient proton-coupled electron transfer (PCET) to produce the methane and isolated ether photoproducts. Singlet and triplet excited-state lifetimes were measured in CH3OH and CD3OD to probe the kinetic isotope effects (KIEs). The resulting KIEs are small, implying that the kinetic bottleneck is due to the C–B bond scission rather than the subsequent PCET. The introduction of a methoxy group in the meso-phenoxy substituent redirects the photosubstitution toward the meso-position. The corresponding regiochemistry was explained computationally. On elongating the C–O bonds in the S1 state, it is found that the unproductive conical intersection is encountered much earlier for the alkyl–O bond than for the phenyl–O bond. The current findings are valuable for the rational design of new BODIPY photocages with tailored biological applications.
硼在BODIPY光笼中观位上的竞争光裂解
BODIPY光笼(光可切割保护基团)引起了人们的兴趣,因为它们可以在可见光激发下释放生物活性货物。我们对选定的BODIPY光笼进行了理论和实验相结合的研究,以阐明硼位和介位竞争光裂解的机理。根据计算,前一反应涉及B-C键的延伸,产生紧密的硼阳离子和甲基阴离子。这些离子被CH3OH拦截,使质子耦合电子转移(PCET)有效地产生甲烷和分离的醚光产物。在CH3OH和CD3OD中测量了单线态和三重态激发态寿命,以探测动力学同位素效应(KIEs)。由此产生的KIEs很小,这意味着动力学瓶颈是由于C-B键的断裂而不是随后的PCET。在中邻苯氧基取代基中引入甲氧基,使光取代指向中位。对相应的区域化学进行了计算解释。在S1态下拉长C-O键时,发现烷基- o键比苯基- o键更早地遇到非生产锥形交叉。目前的研究结果对合理设计具有定制生物学应用的新型BODIPY光笼具有重要价值。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


