Electrochemical Oxidative Cascade Cyclization of Alkenyl Alcohols with External Nucleophiles to Access Amino- and Hydroxy-Functionalized O-Heterocycles
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
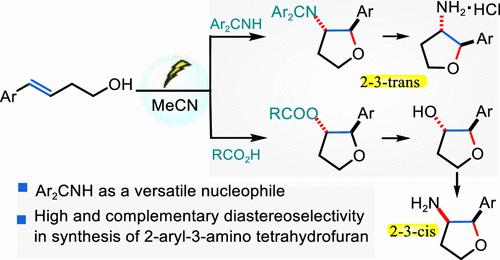
A convenient electrochemical oxidative cascade cyclization of alkenes equipped with pendant alcohols with general nucleophiles was developed. Using readily available diarylmethanimine and carboxylic acids as nucleophilic sources, a broad range of internal alkene and terminal alkene substrates could produce RCO2- and Ar2CN-functionalized O-heterocycles in moderate to high yields without the requirement for external oxidants and metals. These resulting products can subsequently be hydrolyzed to yield valuable NH2- and OH-functionalized tetrahydrofurans and tetrahydropyranes under mild conditions. Importantly, the efficient conversion of secondary alcohol products to amines with complete inversion of configuration enhances the methodology, enabling the construction of 2-aryl-3-amino tetrahydrofuran with high and complementary diastereoselectivity.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


