Generation and Application of All Possible Conformations of Cyclic Tryptophan within and beyond Post-translational Modification
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
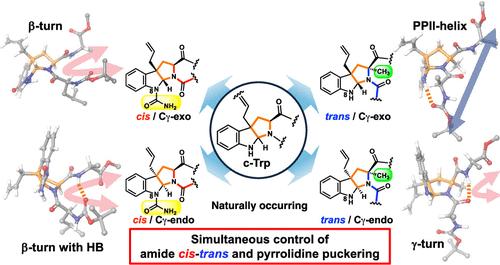
Isoprenylation of the indole C3-position of tryptophan accompanied by cyclization (c-Trp) is one of the most attractive post-translational modifications because of C–C bond formation and drastic conformational alteration. As the modification generates two stereoisomers of the 6/5/5-fused ring system and consequently, a mixture of four possible conformations as considered in proline, it is expected to influence the biological activity in Bacillus quorum sensing pheromone ComX containing the c-Trp residue. In this study, the simultaneous control of the amide cis–trans equilibrium and pyrrolidine ring puckering was achieved by utilizing an N-carbamoylated and α-methylated 6/5/5-fused ring system. Furthermore, the conformationally defined tripeptides containing the c-Trp residue were utilized to examine the relationship between the biological activity and the conformation of the ComX pheromone. Several mimics showed high bioactivity, and more biologically active ComX mimics were created to reinforce the CH-π interaction of the c-Trp and the adjacent aromatic residue.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


