Synthesis of Aminated C-3 Aryloylated Benzofuran, Furopyridine, Benzothiophene, and Indole Derivatives from 1,6-Enyne and N-Aminopyridinium Salt in Visible Light
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
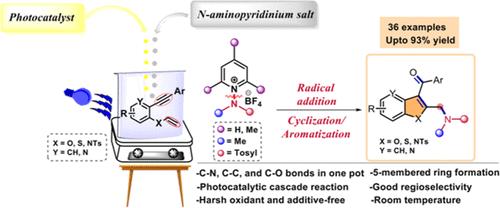
We report a visible-light-assisted tandem oxidative 5-exo-dig cyclization of 1,6-enynes for the synthesis of aminated C-3 aryloylated benzofuran, furopyridine, benzothiophene, and indole derivatives. The nitrogen-centered radical generated in situ from N-aminopyridinium salt initiates the consecutive formation of C–N, C–C, and C–O bonds. The methodology exhibits good functional group tolerance and regioselectivity, furnishing products in good to excellent yields at room temperature. Preliminary biological screening of synthesized molecules reveals their potential as anticancer agents.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


