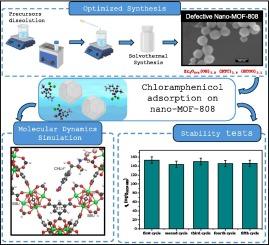
Enhanced adsorptive removal of chloramphenicol from water by highly defective MOF-808 nanocrystals fine-tuned with reliable synthesis strategy: Mechanism insight by equilibrium, kinetics and molecular dynamics simulations
IF 13.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
This study proposes for the first time the use of nanoscale MOF-808 for the adsorptive removal of the antibiotic chloramphenicol from aqueous solutions. A reliable and robust synthesis strategy is used to downsize MOF-808 to the nanoscale, resulting in nanocrystals with an octahedral morphology (average size 80 nm) and a significantly high specific surface area (SSA around 2100 m2g−1). The determination of the actual formula of the highly defective nano-MOF is assessed by thermogravimetric analysis. Adsorption of chloramphenicol onto nano-MOF-808 is investigated by varying initial drug concentration, adsorbent dosage, and pH. Nearly complete chloramphenicol removal (99 %) is achieved within 60 min when treating an aqueous solution at the pollutant initial concentration of 50 mg/L with 0.25 g/L nano-MOF-808 dosage and at the spontaneous pH of drug aqueous solution (pH = 6). Kinetic and isotherm studies reveal pseudo-second-order kinetics and Langmuir isotherm as the best-fit models. The adsorption energy estimated by the Dubinin-Radushkevich model indicates that drug adsorption is mainly characterized by the establishment of weak interactions, typical of physical adsorption. The experimental data obtained at different pHs are discussed in conjunction with molecular dynamics simulations, showing that the chloramphenicol is mainly adsorbed on the external surface of the nano-MOF-808 and that the rate-limiting step is the diffusion of drug molecules through the boundary layer surrounding the nano-MOF whose charge, altered by the pH of the solution, can promote or inhibit the drug intake. Finally, the prepared nano-MOF-808 shows high stability since the adsorption performances did not worsen after five reuse cycles
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


