Single-cell RNA sequencing-derived signatures define response patterns to atezolizumab + bevacizumab in advanced hepatocellular carcinoma
IF 26.8
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
Abstract
Background & Aims
The combination of atezolizumab and bevacizumab (atezo+bev) is the current standard of care for advanced hepatocellular carcinoma (HCC), providing a median overall survival (OS) of 19.2 months. Here, we aim to uncover the underlying cellular processes driving clinical benefit vs. resistance to atezo+bev.
Methods
We harnessed the power of single-cell RNA sequencing in advanced HCC to derive gene expression signatures recapitulating 21 cell phenotypes. These signatures were applied to 422 RNA-sequencing samples of patients with advanced HCC treated with atezo+bev (n = 317) vs. atezolizumab (n = 47) or sorafenib (n = 58) as comparators.
Results
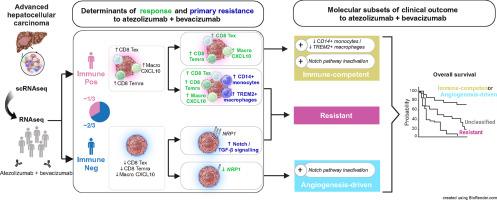
We unveiled two distinct patterns of response to atezo+bev. First, an immune-mediated response characterised by the combined presence of CD8+ T effector cells and pro-inflammatory CXCL10+ macrophages, representing an immune-rich microenvironment. Second, a non-immune, angiogenesis-related response distinguishable by a reduced expression of the VEGF co-receptor neuropilin-1 (NRP1), a biomarker that specifically predicts improved OS upon atezo+bev vs. sorafenib (p = 0.039). Primary resistance was associated with an enrichment of immunosuppressive myeloid populations, namely CD14+ monocytes and TREM2+ macrophages, and Notch pathway activation. Based on these mechanistic insights we define "Immune-competent" and "Angiogenesis-driven" molecular subgroups, each associated with a significantly longer OS with atezo+bev vs. sorafenib (p of interaction = 0.027), and a “Resistant” subset.
Conclusion
Our study unveils two distinct molecular subsets of clinical benefit to atezolizumab plus bevacizumab in advanced HCC (“Immune-competent” and “Angiogenesis-driven”) as well as the main traits of primary resistance to this therapy, thus providing a molecular framework to stratify patients based on clinical outcome and guiding potential strategies to overcome resistance.
Impact and implications
Atezolizumab + bevacizumab (atezo+bev) is standard of care in advanced hepatocellular carcinoma (HCC), yet molecular determinants of clinical benefit to the combination remain unclear. This study harnesses the power of single-cell RNA sequencing, deriving gene expression signatures representing 21 cell subtypes in the advanced HCC microenvironment. By applying these signatures to RNA-sequencing samples, we reveal two distinct response patterns to atezo+bev and define molecular subgroups of patients (“Immune-competent” and “Angiogenesis-driven” vs. “Resistant”) with differential clinical outcomes upon treatment with atezo+bev, pointing towards the role of immunosuppressive myeloid cell types and Notch pathway activation in primary resistance to atezo+bev. These results may help refine treatment strategies and improve outcomes for patients with advanced HCC, while also guiding future research aimed at overcoming resistance mechanisms.
单细胞RNA序列衍生的特征定义了晚期肝细胞癌对atezolizumab +贝伐单抗的反应模式
背景,atezolizumab和bevacizumab联合(atezo+bev)是目前晚期肝细胞癌(HCC)的标准治疗方案,提供19.2个月的中位总生存期(OS)。在这里,我们的目标是揭示驱动atezo+bev临床获益与耐药的潜在细胞过程。方法利用单细胞RNA测序技术在晚期HCC中获得21种细胞表型的基因表达特征。这些特征被应用于422例晚期HCC患者的rna测序样本,这些患者使用atezo+bev (n=317)与atezolizumab (n=47)或索拉非尼(n=58)作为比较。结果揭示了两种不同的atezo+bev反应模式。首先,以CD8+ T效应细胞和促炎CXCL10+巨噬细胞的联合存在为特征的免疫介导反应,代表了一个免疫丰富的微环境。其次,非免疫性血管新生相关反应,可通过VEGF共受体neuropilin-1 (NRP1)的表达降低来区分,NRP1是一种生物标志物,可特异性预测atezo+bev与索拉非尼相比改善OS (p = 0.039)。原发性耐药与免疫抑制髓细胞群(CD14+单核细胞和TREM2+巨噬细胞)的富集以及Notch通路的激活有关。基于这些机制的见解,我们定义了“免疫能力”和“血管生成驱动”分子亚群,与索拉非尼相比,atezo+bev的每个亚群与更长的生存期相关(相互作用p = 0.027),以及一个“耐药”亚群。我们的研究揭示了atezolizumab联合贝伐单抗治疗晚期HCC的两种不同的临床获益分子亚群(“免疫能力”和“血管生成驱动”)以及对该疗法的原发性耐药的主要特征,从而提供了基于临床结果对患者进行分层的分子框架,并指导了克服耐药的潜在策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Hepatology
医学-胃肠肝病学
CiteScore
46.10
自引率
4.30%
发文量
2325
审稿时长
30 days
期刊介绍:
The Journal of Hepatology is the official publication of the European Association for the Study of the Liver (EASL). It is dedicated to presenting clinical and basic research in the field of hepatology through original papers, reviews, case reports, and letters to the Editor. The Journal is published in English and may consider supplements that pass an editorial review.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


