Conformational Analysis of Swallowtail Motifs in Porphyrins
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
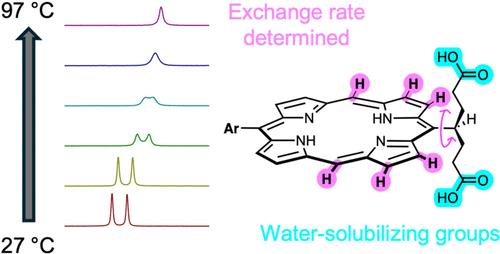
Aqueous solubilization of porphyrins, often accomplished with appended polar aryl groups, can also be achieved with symmetrically branched alkyl (i.e., swallowtail) groups terminated with polar moieties. Here, carboxylic acids are employed as termini (versus prior phosphono- or phosphoester termini) in designs of trans-AB-porphyrins bearing a single swallowtail group (A) or trans-A2-porphyrins bearing two swallowtail groups. Variable-temperature 1H NMR studies (−60 to 97 °C) revealed that the 4-heptanedioic acid group at the meso-position of the free base porphyrin rotates with rate constant 5 s–1 (310 K) and Arrhenius energy barrier Ea = 11.5 kcal/mol, whereas an isopropyl group undergoes rotation ∼1000-times faster (k = 5770 s–1). The interconversion is sufficiently fast that conformational diastereomers, as when two such swallowtail groups are present in a trans-A2-porphyrin, would not be isolable at room temperature (Class I atropisomers). DFT calculations (4 porphyrins containing the isopropyl or 4-heptanedioic acid groups) showed that the lowest energy conformer contains the swallowtail C–H unit in the plane of the porphyrin. The presence of one or two 4-heptanedioic acid moieties imparted solubility of the porphyrin in phosphate-buffered saline (PBS). The results suggest applications in the life sciences where compact, aqueous-soluble porphyrins are desired.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


