Organogel Polymer Electrocatalysts for Two-Electron Oxygen Reduction
IF 13
2区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
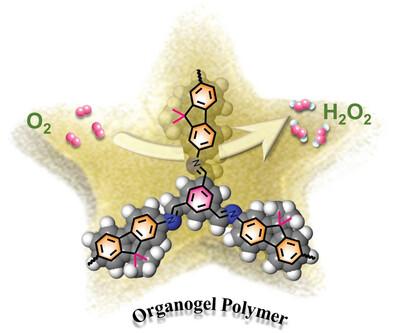
Polymer gels, renowned for unparalleled chemical stability and self-sustaining properties, have garnered significant attention in electrocatalysis. Notably, organic polymer gels that exhibit temperature sensitivity and incorporate suitable polar nonvolatile liquids, enhance electronic conductivity, and impart distinct morphological features, but remain largely unexplored as electrocatalysts for oxygen reduction reaction (ORR). To address this issue, an innovative strategy is proposed for synergistic modulation of the rigidity of mainchain molecular skeleton and length of alkyl sidechains, enabling the development of organogel polymers with a sol–gel temperature-sensitive phase transition that promises high selectivity and enhanced activity in electrocatalytic processes. Notably, the shortening of alkyl sidechain length can significantly affect the gelation behavior and internal microstructure of the catalyst, which modifies the electron state, ultimately impacting the catalytic activity of the gel polymer catalysts. In particular, phenyl-containing Ph-FL1 with short alkyl sidechains demonstrates outstanding 2e− ORR activity in alkaline medium, achieving a remarkable hydrogen peroxide (H2O2) selectivity of 98.6% with an impressive yield of 4.08 mol g−1 h−1. This performance surpasses most metal-free carbon-based electrocatalysts. Through theoretical calculation, the carbon atom (site-3) of C═N group is identified as potential active sites, representing a significant advancement toward designing cost-effective and efficient ORR electrocatalysts.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Small
工程技术-材料科学:综合
CiteScore
17.70
自引率
3.80%
发文量
1830
审稿时长
2.1 months
期刊介绍:
Small serves as an exceptional platform for both experimental and theoretical studies in fundamental and applied interdisciplinary research at the nano- and microscale. The journal offers a compelling mix of peer-reviewed Research Articles, Reviews, Perspectives, and Comments.
With a remarkable 2022 Journal Impact Factor of 13.3 (Journal Citation Reports from Clarivate Analytics, 2023), Small remains among the top multidisciplinary journals, covering a wide range of topics at the interface of materials science, chemistry, physics, engineering, medicine, and biology.
Small's readership includes biochemists, biologists, biomedical scientists, chemists, engineers, information technologists, materials scientists, physicists, and theoreticians alike.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


