NMR resonance assignment of a ligand-binding domain of ephrin receptor A2
IF 0.6
4区 生物学
Q4 BIOPHYSICS
引用次数: 0
Abstract
Ephrin receptors regulate intercellular communication and are thus involved in tumor development. Ephrin receptor A2 (EphA2), in particular, is overexpressed in a variety of cancers and is a proven target for anti-cancer drugs. The N-terminal ligand-binding domain of ephrin receptors is responsible for the recognition of their ligands, ephrins, and is directly involved in receptor activation. Here, we report on the complete 1H, 15N and 13C NMR chemical shift assignment of EphA2 ligand binding domain that provides the basis for NMR-assisted drug design.
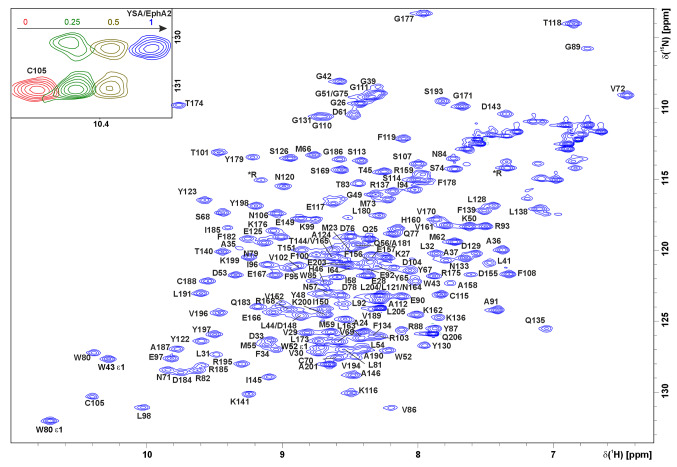
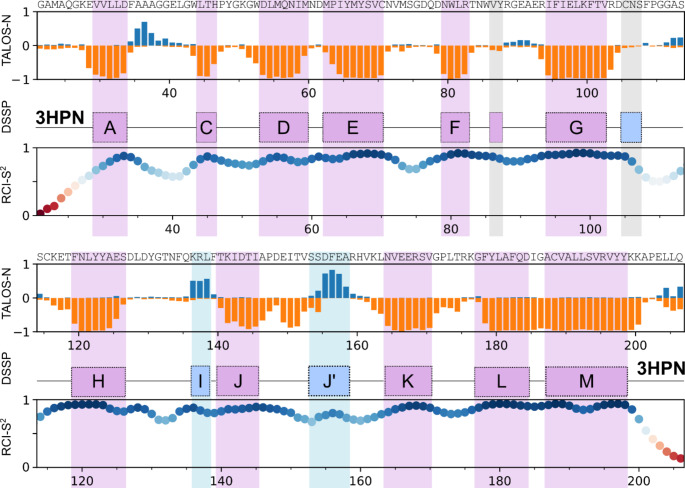
ephrin受体A2配体结合域的核磁共振配位。
Ephrin受体调节细胞间通讯,因此参与肿瘤的发展。尤其是Ephrin受体A2 (EphA2),在多种癌症中过度表达,是抗癌药物的靶点。ephrin受体的n端配体结合域负责其配体,ephrin的识别,并直接参与受体的激活。在这里,我们报道了EphA2配体结合域的完整1H, 15N和13C NMR化学位移分配,为核磁共振辅助药物设计提供了基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biomolecular NMR Assignments
生物-光谱学
CiteScore
1.70
自引率
11.10%
发文量
59
审稿时长
6-12 weeks
期刊介绍:
Biomolecular NMR Assignments provides a forum for publishing sequence-specific resonance assignments for proteins and nucleic acids as Assignment Notes. Chemical shifts for NMR-active nuclei in macromolecules contain detailed information on molecular conformation and properties.
Publication of resonance assignments in Biomolecular NMR Assignments ensures that these data are deposited into a public database at BioMagResBank (BMRB; http://www.bmrb.wisc.edu/), where they are available to other researchers. Coverage includes proteins and nucleic acids; Assignment Notes are processed for rapid online publication and are published in biannual online editions in June and December.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


