New Insights into CO2 Electroreduction in Acidic Seawater
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
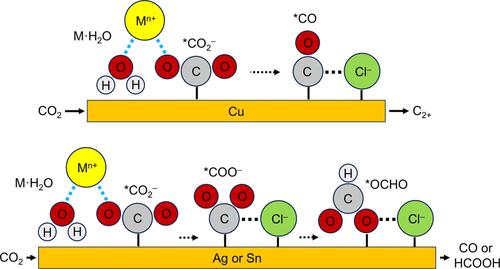
The electrochemical CO2 reduction reaction (CO2RR) is of great importance to produce valuable chemicals. In conventional alkaline and “acid + salts”-based CO2RR, the aqueous electrolyte normally needs to be refreshed due to the gradually more neutral feature of pH during electrolysis operation. Therefore, both solutes and deionized (DI) water in electrolytes are required to be regenerated regularly. In this work, acidic seawater (pH < 2) was used as a low-cost but efficient electrolyte for CO2RR without salt addition. The Faradaic efficiencies (FEs) and partial current densities of C2+ on typical copper in the “H2SO4 in raw seawater” electrolyte are comparable with those for conventional “KOH in DI water” and much higher than those for “H2SO4 + salts” systems. Moreover, single-pass carbon efficiencies (SPCEs) in acidic seawater are significantly higher than the values in alkaline DI water. Such an abnormal phenomenon was also demonstrated for CO and HCOOH generation on typical silver and tin catalysts, respectively. In situ Raman spectroscopy and controlled experiments revealed that metal (denoted as M) cations in seawater ensure a higher concentration of M·H2O species, which improve interactions with *CO2–, while Cl– anions enhance the adsorption strength of key CO2RR intermediates (namely, *CO on copper, *COO– on silver, and *OCHO on tin). Through these interactions with water molecules and CO2RR intermediates, such free but functional ions in seawater play a highly important role in promoting selectivity and activity for CO2RR, as well as SPCE in acidic seawater. Furthermore, using acidic seawater as an alternative CO2RR electrolyte has significant economic and ecological benefits compared with traditional alkaline DI water electrolytes.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


