Impact of Surface Adsorption on DNA Structure and Stability: Implications for Environmental DNA Interactions with Iron Oxide Surfaces
IF 3.7
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
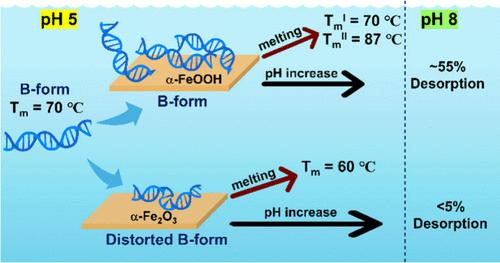
Environmental DNA (eDNA), i.e., DNA found in the environment, can interact with various geochemical surfaces, yet little is known about these interactions. Mineral surfaces may alter the structure, stability, and reactivity of eDNA, impacting the cycling of genetic information and the reliability of eDNA-based detection tools. Understanding how eDNA interacts with surfaces is crucial for predicting its fate in the environment. In this study, we examined the surface interaction and stability of herring testes DNA, a model system for eDNA, on two common iron oxide phases present in the environment: α-FeOOH (goethite) and α-Fe2O3 (hematite). Utilizing spectroscopic probes, including attenuated total reflection Fourier-transform infrared (ATR-FTIR) and UV–vis spectroscopy, we quantified the DNA adsorption capacity at pH 5 and determined its secondary structure. DNA adsorbed irreversibly at pH 5 and 25 °C, primarily through its phosphate groups, and retained the solution-phase B-form structure. However, the infrared data also indicated some distortion of the B-form likely due to additional interactions between nitrogenous bases when adsorbed on the α-Fe2O3 particle surfaces. The distortion in the double helical structure of adsorbed DNA on α-Fe2O3 led to a lower melting temperature (Tm) of 60 °C compared to 70 °C for DNA in solution. In contrast, DNA adsorbed on α-FeOOH melted at higher temperatures relative to solution-phase DNA and in two distinct phases. Upon testing adsorbed DNA stability at higher pH values, there were distinct differences between the two iron oxide phases. For α-FeOOH, nearly 50% of the DNA desorbed from the surface when the solution pH changed from 5 to 8, while less than 5% desorbed from α-Fe2O3 under the same conditions. Overall, these findings underscore the importance of mineral-specific eDNA–surface interactions and their role in adsorbed eDNA stability, in terms of DNA melting and the impact of solution-phase pH changes.
表面吸附对DNA结构和稳定性的影响:环境DNA与氧化铁表面相互作用的影响
环境 DNA(eDNA),即在环境中发现的 DNA,可与各种地球化学表面相互作用,但人们对这些相互作用知之甚少。矿物表面可能会改变 eDNA 的结构、稳定性和反应性,从而影响遗传信息的循环和基于 eDNA 的检测工具的可靠性。了解 eDNA 与表面的相互作用对于预测其在环境中的命运至关重要。在本研究中,我们研究了鲱鱼睾丸 DNA(eDNA 的模型系统)在环境中常见的两种氧化铁相:α-FeOOH(鹅铁矿)和 α-Fe2O3(赤铁矿)上的表面相互作用和稳定性。利用光谱探针,包括衰减全反射傅立叶变换红外光谱(ATR-FTIR)和紫外-可见光谱,我们量化了 DNA 在 pH 值为 5 时的吸附能力,并确定了其二级结构。DNA 在 pH 值为 5 和 25 °C 时主要通过其磷酸基团进行不可逆吸附,并保留了溶液相的 B 型结构。不过,红外数据也表明,B 型结构发生了一些变形,这可能是由于含氮碱基吸附在 α-Fe2O3 颗粒表面时发生了额外的相互作用。吸附在 α-Fe2O3 上的 DNA 的双螺旋结构发生扭曲,导致其熔化温度(Tm)降低到 60 °C,而溶液中 DNA 的熔化温度为 70 °C。与此相反,吸附在 α-FeOOH 上的 DNA 熔化温度比溶液相的 DNA 熔化温度要高,而且有两个不同的阶段。在测试吸附 DNA 在较高 pH 值下的稳定性时,两种氧化铁相之间存在明显差异。对于 α-FeOOH,当溶液 pH 值从 5 变为 8 时,近 50% 的 DNA 从表面脱附,而在相同条件下,从 α-Fe2O3 脱附的 DNA 不到 5%。总之,这些发现强调了矿物特异性 eDNA 与表面相互作用的重要性,以及它们在 DNA 熔化和溶液相 pH 变化影响吸附的 eDNA 稳定性方面的作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


