Engineering a Binding Peptide for Oriented Immobilization and Efficient Bioelectrocatalytic Oxygen Reduction of Multicopper Oxidases
IF 8.3
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
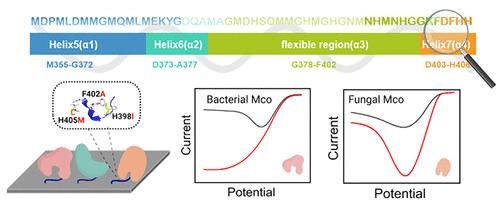
Enzymatic fuel cells (EFCs) are emerging as promising technologies in renewable energy and biomedical applications, utilizing enzyme catalysts to convert the chemical energy of renewable biomass into electrical energy, known for their high energy conversion efficiency and excellent biocompatibility. Currently, EFCs face challenges of poor stability and catalytic efficiency at the cathodes, necessitating solutions to enhance the oriented immobilization of multicopper oxidases for improved heterogeneous electron transfer efficiency. This study successfully identified a surface-binding peptide (SBP, 13 amino acids) derived from a methionine-rich fragment (MetRich, 53 amino acids) in E. coli CueO through semirational design. The first phase of engineering focused on the structural characteristics of MetRich, pinpointing fragment N394-H406 (SBP 1.0, corresponding to variant CueO-M12) as the key region dominating the binding. Subsequent site-saturation mutagenesis, combined with electrochemical screening, yielded three variants, and among them, the variant CueO-M12-1 (CueO-M12 H398I) exhibited a more uniform favorable orientation with a 1.38-fold increase in current density. Further electrocatalytic kinetics analysis revealed a significant 21.2-fold improvement in kinetics current density (Jk) compared with that of CueO-WT, leading to the development of SBP 2.0. When SBPs were fused to laccase from Bacillus pumilus (BpL) and fungal bilirubin oxidase from Myrothecium verrucaria (MvBOD), respectively, they transformed a sluggish adsorption process into a rapid and oriented one. In addition, compared with SBP 1.0, SBP 2.0 endows BpL and MvBOD with enhanced electrocatalytic capabilities for oxygen reduction and glucose/O2 EFC performance. The engineered SBPs are promising for serving as a versatile “glue” to enable the immobilization of oxidoreductases in an oriented manner, which leads to a breakthrough in bioelectrocatalysis and thereby overcoming the current bottleneck of EFCs.
为多铜氧化酶的定向固定和高效生物电催化氧还原设计一种结合肽
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


