Sustainable Synthesis of Substituted 1,3,5-Triazines by [ONO]-Pincer-Supported Nickel(II) Complexes via an Acceptorless Dehydrogenative Coupling Strategy
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A facile, cost-effective, and sustainable synthesis of substituted triazines from primary alcohols by newly synthesized nickel pincer-type complexes (1–3) has been described. Herein, we report the synthesis of a set of three well-defined Ni(II) O^N^O pincer-type complexes, structurally characterized by analytical, spectral, and X-ray diffraction techniques. Further, the nickel complexes are explored as efficient catalysts (4 mol %) for the construction of 2,4,6-substituted 1,3,5-triazines from readily available alcohols via an acceptorless dehydrogenative coupling (ADC) strategy. A wide range of substituted triazine derivatives (33 examples) has been synthesized from the coupling of alcohols and benzamidine/guanidine hydrochloride with a maximum isolated yield of 92% under mild conditions, with eco-friendly H2O and H2 gas as the only byproducts. A plausible mechanism has been proposed based on a sequence of control experiments. Interestingly, the short synthesis of the antiulcer drug irsogladine and the large-scale synthesis of 2,4-diphenyl-6-(p-tolyl)-1,3,5-triazine highlight the convenience of the current methodology.
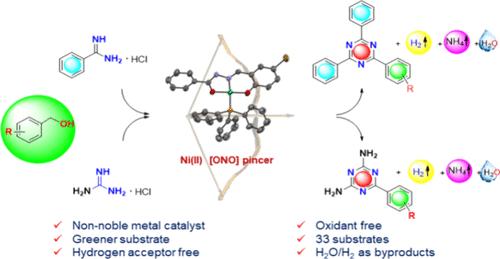
无受体脱氢偶联镍螯合物可持续合成1,3,5-三嗪类取代物
新合成的镍钳型配合物(1-3)可以方便、经济、可持续地从伯醇中合成取代三嗪。在此,我们报告了一组三种定义明确的 Ni(II) O^N^O 钳子型配合物的合成,并通过分析、光谱和 X 射线衍射技术对其结构进行了表征。此外,研究人员还将这些镍络合物作为高效催化剂(4 摩尔%),通过无受体脱氢偶联(ADC)策略,从现成的醇中合成 2,4,6-取代的 1,3,5-三嗪。在温和的条件下,通过醇和苯甲脒/盐酸胍的偶联合成了多种取代的三嗪衍生物(33 例),最高分离产率达 92%,唯一的副产物是环保的 H2O 和 H2 气体。在一系列对照实验的基础上,提出了一个合理的机制。有趣的是,抗溃疡药物伊索格拉定的简短合成和 2,4-二苯基-6-(对甲苯基)-1,3,5-三嗪的大规模合成凸显了当前方法的便利性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


