Overcoming radiation-induced PD-L1 upregulation by novel gadolinium-palladium nanoplatforms for enhanced tumor radio-immunotherapy
IF 13.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
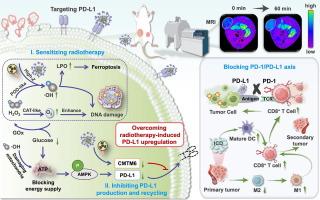
Low-dose radiotherapy can lead to upregulation of PD-L1 expression in tumor cells, limiting the effectiveness of radio-immunotherapy. Currently, anti-PD-L1 drugs only disrupt the interaction of the PD-1/PD-L1 axis on the cell surface, without considering the immune regulatory ability of intracellular PD-L1. Additionally, antibody-bound PD-L1 can lead to internalization and recycling, allowing tumor cells to regain immune suppression. In this study, a novel PD-L1-affibody (ZPD-L1)-grafted gadolinium-palladium nanoplatform incorporated with glucose oxidase (GOx) (GPGP) was fabricated to improve tumor-targeted magnetic resonance imaging (MRI) and synergistic radio-immunotherapy. GPGP with catalase- and peroxidase-like activity could catalyze the generation of oxygen to relieve tumor hypoxia and the generation of •OH to boost ferroptosis, respectively. GOx could enhance the nanoplatform’s dual nanozyme activity in a cascade, further sensitizing radiotherapy and intensifying subsequent immunogenic cell death. Crucially, GPGP induced AMPK phosphorylation and downregulated CMTM6, inhibiting intracellular PD-L1 production and blocking its transport to the cell membrane, thereby overcoming low-dose radiotherapy-induced PD-L1 upregulation. Systemic delivery of GPGP also demonstrated notable MRI contrast enhancement, providing precise imaging guidance for determining the optimal timing for radiotherapy. Therefore, GPGP has shown great potential in achieving effective MRI and collaborative tumor radio-immunotherapy.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


