Why Mixed Halides in 2D Chiral Perovskites Weaken Chirality-Induced Spin Selectivity
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
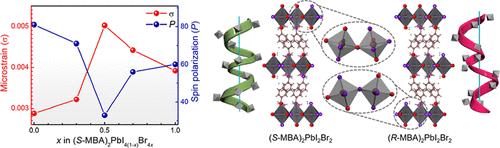
2D Ruddlesden–Popper (RP) perovskites, upon inclusion of a chiral amine, exhibit chirality-induced spin selectivity (CISS). Although alloying at the halogen site in MBA-based RPs (MBA: methylbenzylammonium) is one of the suitable routes to tune the CISS effect, the mixed-halide RP perovskites exhibited complete suppression of chirality when probed through circular dichroism (CD). Here, we present the CISS effect in a series of mixed-halide RP perovskites. We show that photoinduced halide segregation is the origin for the apparent chirality suppression. The spin-dependent charge transport was evidenced through magnetic-conducting atomic force microscopy (mc-AFM) studies and magnetoresistance (MR) measurements. The mc-AFM results show that in (R/S-MBA)2PbI4(1–x)Br4x, the CISS effect decreases with bromide inclusion, nonmonotonically; the microstrain developed in the lattice and the spin-polarized charge transport are found to be correlated. Such a behavior has been explained through an inhomogeneity in the strength of the hydrogen bond between the organic moieties and halogens in the inorganic framework of the compounds. Our results further inferred that the hydrogen-bond-induced coupling transfers the chirality from the amine to the inorganic sublattice. The work explains the weakened CISS effect in mixed-halide chiral RP perovskites and provides a strategy to tune the spin-polarized charge transport as well.
为什么二维手性钙钛矿中的混合卤化物会削弱手性诱导的自旋选择性
含有手性胺的2D Ruddlesden-Popper (RP)钙钛矿表现出手性诱导的自旋选择性(CISS)。虽然在基于MBA的RP (MBA: methylbenzylammonium)中卤素位点合金化是调整CISS效应的合适途径之一,但通过圆二色性(CD)探测时,混合卤化物RP钙钛矿表现出完全的手性抑制。在这里,我们提出了一系列混合卤化物RP钙钛矿的CISS效应。我们发现光致卤化物偏析是明显手性抑制的根源。通过磁导原子力显微镜(mc-AFM)研究和磁电阻(MR)测量证明了自旋相关的电荷输运。mc-AFM结果表明,在(R/S-MBA)2PbI4(1-x)Br4x中,CISS效应随溴化物的加入而非单调降低;在晶格中产生的微应变与自旋极化电荷输运是相关的。这种行为可以通过化合物的无机骨架中有机部分和卤素之间氢键强度的不均匀性来解释。我们的结果进一步推断,氢键诱导的偶联将手性从胺转移到无机亚晶格。该研究解释了混合卤化物手性RP钙钛矿中减弱的CISS效应,并提供了一种调整自旋极化电荷输运的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


