Hydrogel-Assisted Robust Supraparticles Evolved from Droplet Evaporation
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
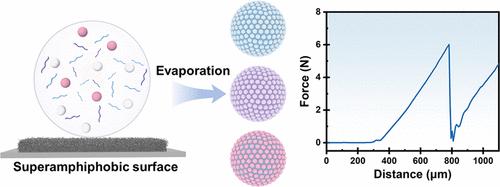
Supraparticles, formed through the self-assembly of nanoparticles, are promising contenders in catalysis, sensing, and drug delivery due to their exceptional specific surface area and porosity. However, their mechanical resilience, especially in dimensions spanning micrometers and beyond, is challenged by the inherently weak interactions among their constituent building blocks, significantly constraining their broad applicability. Here, we have exploited a robust supraparticle fabrication strategy by integrating hydrogel components into the assembly system and evaporating on the superamphiphobic surface. The resultant SiO2/SA (sodium alginate) supraparticles, achieved by evaporating a 15% volume fraction dispersion of SiO2 nanoparticles containing 18.46 mg/mL of sodium alginate and subsequently cross-linking with Ca2+, demonstrate mechanical robustness with a fracture force of 6.04 N, representing a mechanical strength enhancement of 60 times higher than that prior to the incorporation of the hydrogel component. The supraparticles maintain their original morphology after 30 min of ultrasonic treatment (200 W), demonstrating mechanical stability. This method exhibits generalizability, enabling the customization of supraparticles with various building blocks and hydrogel backbone materials. Based on such a methodology, we have synthesized enzyme-carrying supraparticles, further expanding the potential applications in intricate cascade reactions. The encapsulated glucose oxidase and horseradish peroxidase maintained their inherent reactivity, and such hydrogel-assisted robust supraparticles exhibited exceptional performance in accurate glucose assays, indicating great practical application in biocatalysis.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


