Highly stable biochar-encapsulated CoTi@BC nanocatalysts for lignin hydrogenolysis
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Valorization of renewable lignin toward value-added fuels and chemicals can improve the economies of biorefinery. However, maintaining catalyst stability and preventing metal aggregation under the certain conditions of lignin hydrogenolysis remains a key challenge. Herein, hydrogenolysis of corncob enzymatic lignin was investigated using biochar-encapsulated CoTi@BC catalysts at the reaction temperature of 250 °C. Co1Ti0.5@BC catalyst with the addition of Ti species outperforms Co@BC catalyst, resulting in 82.5 % lignin liquefaction degree and 23.7 wt% yield of monophenols. Besides, the catalytic stability of Co1Ti0.5@BC catalyst is outstanding in the lignin hydrogenolysis, where almost no activity loss occurred after four recycle runs. Catalyst characterization suggests that the addition of moderate amounts of Ti species changed the reduction temperature of Co species and the interaction between metal sites and carbon layer. The uniform distribution of Ti species improves the dispersion of Co metal particles, and the carbon layer can protect the surface of metal nanoparticles from oxidation, thus maintaining the stability and the activity of Co metal sites. Furthermore, the mechanism of lignin hydrogenolysis with CoTi@BC catalysts was investigated based on the results of benzyloxyphenol hydrogenolysis. These findings demonstrate the unique advantages of biochar-encapsulated metal particles for efficient C-O bond cleavage and offer valuable insights for advancing lignin valorization and sustainable biorefinery development.
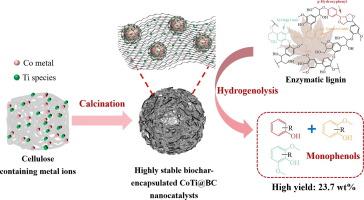
用于木质素氢解的高稳定性生物炭包封 CoTi@BC 纳米催化剂
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


