Synaptic basis of feature selectivity in hippocampal neurons
IF 50.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
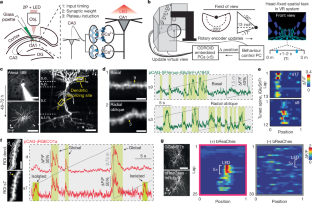
A central question in neuroscience is how synaptic plasticity shapes the feature selectivity of neurons in behaving animals1. Hippocampal CA1 pyramidal neurons display one of the most striking forms of feature selectivity by forming spatially and contextually selective receptive fields called place fields, which serve as a model for studying the synaptic basis of learning and memory. Various forms of synaptic plasticity have been proposed as cellular substrates for the emergence of place fields. However, despite decades of work, our understanding of how synaptic plasticity underlies place-field formation and memory encoding remains limited, largely due to a shortage of tools and technical challenges associated with the visualization of synaptic plasticity at the single-neuron resolution in awake behaving animals. To address this, we developed an all-optical approach to monitor the spatiotemporal tuning and synaptic weight changes of dendritic spines before and after the induction of a place field in single CA1 pyramidal neurons during spatial navigation. We identified a temporally asymmetric synaptic plasticity kernel resulting from bidirectional modifications of synaptic weights around the induction of a place field. Our work identified compartment-specific differences in the magnitude and temporal expression of synaptic plasticity between basal dendrites and oblique dendrites. Our results provide experimental evidence linking synaptic plasticity to the rapid emergence of spatial selectivity in hippocampal neurons, a critical prerequisite for episodic memory. A temporally asymmetric synaptic plasticity kernel results from bidirectional modifications of synaptic weights around the induction of a place field.

海马神经元特征选择性的突触基础
神经科学的一个核心问题是突触可塑性如何影响行为动物神经元的特征选择性。海马CA1锥体神经元通过形成被称为位置场的空间和情境选择性接受野,显示出最显著的特征选择性形式之一,这是研究学习和记忆的突触基础的模型。不同形式的突触可塑性被认为是位置场出现的细胞基质。然而,尽管经过数十年的研究,我们对突触可塑性如何影响位置场形成和记忆编码的理解仍然有限,这主要是由于缺乏工具和技术挑战,无法在清醒行为动物的单神经元分辨率上可视化突触可塑性。为了解决这个问题,我们开发了一种全光学方法来监测空间导航过程中单个CA1锥体神经元位置场诱导前后树突棘的时空调谐和突触重量变化。我们发现了一个时间上不对称的突触可塑性核,这是由位置场诱导周围突触权重的双向修改引起的。我们的工作确定了基底树突和斜树突之间突触可塑性的大小和时间表达的室特异性差异。我们的研究结果提供了实验证据,证明突触可塑性与海马神经元空间选择性的快速出现有关,而空间选择性是情景记忆的关键先决条件。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


