Stability of the Au/electrolyte interface during hydrogen evolution: A Cyclic Plasmo-Voltammetry study
IF 5.5
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
Metal-electrolyte interfaces are dynamic entities, the potential and electrolyte dependent mobility of the metal atoms leading to surface restructuring with possible dissolution and degradation. In this work, we investigate the stability of the Au/aqueous electrolyte interface with in situ differential Cyclic Plasmo-Voltammetry (dCPV), augmented by ex situ atomic force microscopy and finite differential time domain simulations. We demonstrate that even the onset of hydrogen evolution is accompanied by pronounced morphological changes of the interface which are by far more prominent than those occurring during Au oxidation and reduction. Furthermore, the stability of the interface heavily depends on pH, the degradation of the electrode being considerably stronger in acidic than in neutral electrolyte. In addition, a clear hydrogen adsorption peak was observed in neutral electrolytes during the cathodic scan, which was more pronounced on a freshly prepared Au electrode than on an aged one. The measured dCPVs in acidic and neutral electrolytes can be explained consistently assuming that (1) adsorbed hydrogen is absorbed into the subsurface region of the Au electrode once HER starts; its subsequent removal as molecular hydrogen causes morphological changes; (2) in the presence of metal cations, adsorbed hydrogen is stabilized through the formation of ternary metal hydrides on the gold surface that stabilize the surface Au-H bonds and hinder further absorption of H into the subsurface region as well as the release of hydrogen into the electrolyte.

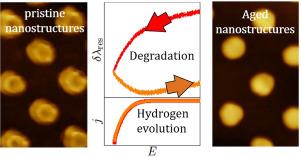
氢演化过程中金/电解质界面的稳定性:循环质谱伏安法研究
金属-电解质界面是动态实体,金属原子的电位和电解质依赖迁移率导致表面重构并可能溶解和降解。在这项工作中,我们使用原位差分循环等离子体伏安法(dCPV),通过非原位原子力显微镜和有限微分时域模拟来研究Au/水电解质界面的稳定性。我们证明,即使是氢演化的开始也伴随着明显的界面形态变化,这种变化远比金氧化和还原过程中发生的变化更为突出。此外,界面的稳定性很大程度上取决于pH值,电极的降解在酸性电解质中比在中性电解质中强得多。此外,在阴极扫描过程中,在中性电解质中观察到一个明显的氢吸附峰,在新制备的Au电极上比在老化的Au电极上更明显。在酸性和中性电解质中测量的dcpv可以一致地解释:(1)一旦HER启动,吸附的氢被吸收到Au电极的亚表面区域;它随后作为氢分子被去除,引起形态变化;(2)在金属阳离子存在的情况下,吸附的氢通过在金表面形成三元金属氢化物来稳定,三元金属氢化物稳定了表面Au-H键,阻碍了H进一步吸收到亚表面区域以及氢释放到电解质中。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


