Enhancing the Electrochemical Energy Storage of Metal–Organic Frameworks: Linker Engineering and Size Optimization
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
The electric conductivity and charge transport efficiency of metal–organic frameworks (MOFs) dictate the effective utilization of built-in redox centers and electrochemical redox kinetics and therefore electrochemical performance. Reticular chemistry and the tunable microcosmic shape of MOFs allow for improving their electric conductivity and charge transfer efficiency. Herein, we synthesized two Ni-MOFs (Ni-tdc-bpy and Ni-tdc-bpe) by the solvothermal reaction of Ni2+ ions with 2,5-thiophenedicarboxylic acid (H2tdc) in the presence of conjugated 4,4′-bipyridyl (bpy) and 1,2-di(4-pyridyl)ethylene (bpe) coligands, respectively. We also synthesized two thinning Ni-MOFs (Ni-tdc-bpy(0.5) and Ni-tdc-bpe(0.5)) by adjusting the amounts of bpy and bpe, respectively. Experimental investigations revealed that linker engineering by tuning the delocalization of the N-donor dipyridyl coligands and size optimization by controlling the amount of the coligand rendered the Ni-MOF with significantly improved electrical conductivity and charge transport efficiency. Among them, Ni-tdc-bpe(0.5) possessing the bpe coligand with more strong delocalization and an optimized size exhibited an enhanced specific capacitance of 650 F g–1 at 0.5 A g–1. Moreover, the hybrid supercapacitor constructed from Ni-tdc-bpe(0.5) and activated carbon delivered an excellent energy density of 33.6 Wh kg–1 at a power density of 232.6 W kg–1.
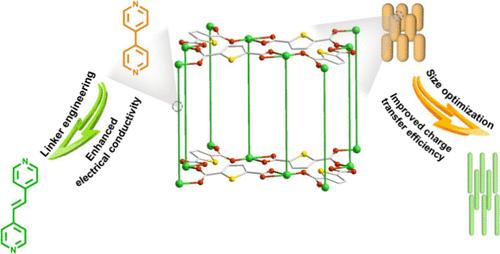
增强金属-有机骨架的电化学储能:连接体工程与尺寸优化
金属有机骨架(MOFs)的电导率和电荷传输效率决定了其内置氧化还原中心的有效利用和电化学氧化还原动力学,从而决定了其电化学性能。mof的网状化学性质和可调的微观形状使其电导率和电荷转移效率得以提高。本文在偶联4,4′-联吡啶基(bpy)和1,2-二(4-吡啶基)乙烯(bpe)的配合体存在下,通过Ni2+离子与2,5-噻吩二羧酸(H2tdc)溶剂热反应,分别合成了Ni-tdc-bpy和Ni-tdc-bpe两种ni - mof。通过调整bpy和bpe的用量,我们分别合成了Ni-tdc-bpy(0.5)和Ni-tdc-bpe(0.5)两种稀释型ni - mof。实验研究表明,通过调节n给体双吡啶配体的离域和通过控制配体的量来优化尺寸,可以使Ni-MOF的电导率和电荷传输效率显著提高。其中,Ni-tdc-bpe(0.5)具有更强的离域和优化尺寸的bpe配体,在0.5 A g-1时的比电容提高到650 F - 1。此外,由Ni-tdc-bpe(0.5)和活性炭构成的混合超级电容器在232.6 W kg-1的功率密度下提供了33.6 Wh kg-1的优异能量密度。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


