Identification of an Isoxazole Derivative as an Antitubercular Compound for Targeting the FadD Enzymes of Mycobacterium tuberculosis
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
FadD32, a fatty acyl-AMP ligase, plays an indispensable role in mycobacterial mycolic acid synthesis and is a validated target for tuberculosis (TB) drug development. The crystal structure of Mycobacterium tuberculosis (Mtb)FadD32 has laid the foundation of structure-based drug discovery against this crucial enzyme. Here, we screened the “isoxazole” scaffold containing molecules against MtbFadD32 and identified a compound 2,4-dibromo-6-[3-(trifluoromethyl)-1,2-oxazol-5-yl]phenol (M1) with specific inhibitory activity against Mtb. Kinetics experiments showed that M1 inhibits MtbFadD32 and MtbFadD28 activity. The transcriptomics response of Mtb disclosed M1-mediated regulation of mycobacterial decisive genes involved in cell wall synthesis, consequently creating unfavorable conditions for Mtb survival. Further, M1 curtails the Mtb survival in infected macrophages and reduces Mtb burden and tubercular granulomas in a chronic infection model of BALB/c mice. Our findings provide an effective chemical scaffold to inhibit MtbFadD32 with the potential to inhibit multiple MtbFadD family of enzymes for further development as a promising candidate for treating TB.
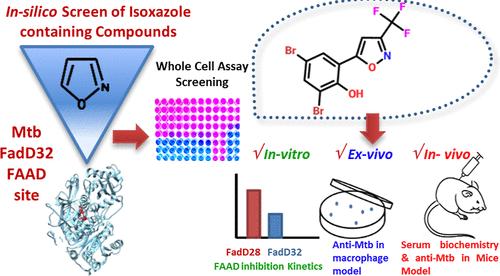
一种针对结核分枝杆菌FadD酶的异恶唑衍生物的抗结核化合物的鉴定
FadD32是一种脂肪酰基- amp连接酶,在分枝杆菌的霉菌酸合成中起着不可或缺的作用,是结核病(TB)药物开发的有效靶点。结核分枝杆菌(Mycobacterium tuberculosis, Mtb)FadD32的晶体结构为针对这种关键酶的基于结构的药物发现奠定了基础。在此,我们筛选了含有MtbFadD32分子的“异恶唑”支架,并鉴定了一种对Mtb具有特异性抑制活性的化合物2,4-二溴-6-[3-(三氟甲基)-1,2-恶唑-5-基]苯酚(M1)。动力学实验表明,M1抑制MtbFadD32和MtbFadD28活性。Mtb的转录组学反应揭示了m1介导的参与细胞壁合成的分枝杆菌决定性基因的调节,从而为Mtb的生存创造了不利的条件。此外,在BALB/c小鼠慢性感染模型中,M1降低了感染巨噬细胞中的结核分枝杆菌存活,减少了结核分枝杆菌负担和结核肉芽肿。我们的研究结果提供了一种有效的化学支架来抑制MtbFadD32,并有可能抑制MtbFadD家族的多种酶,作为治疗结核病的有希望的候选药物进一步开发。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


