Novel steroidal β-carboline derivatives as promising antibacterial candidates against methicillin-resistant Staphylococcus aureus
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
A novel series of steroidal β-carboline quaternary ammonium derivatives (SCQADs) derived from natural cholic acid and its derivatives was designed, synthesized and biologically evaluated against four Gram-positive bacteria for the first time. Most of these derivatives exhibited promising antibacterial activity against the tested strains, particularly, compound 21g displayed strong antibacterial activity against MRSA (MIC = 0.5–1 μg/mL) with low cytotoxicity. Meanwhile, derivative 21g was able to quickly kill Gram-positive bacteria within 0.5 h without inducing bacterial resistance. Preliminary mechanistic explorations indicated that compound 21g destroyed bacterial cell membranes to exert its antibacterial effects. Moreover, 21g exhibited high in vivo efficacy and high survival protection in a mouse skin abscess model. These findings suggested that compound 21g has great potential to develop as an antibacterial agent.
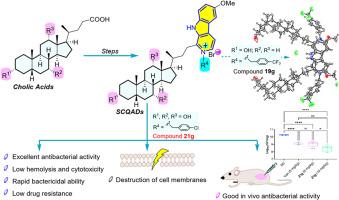

抗甲氧西林金黄色葡萄球菌的新型甾体β-碳碱衍生物
以天然胆酸及其衍生物为原料,设计合成了一系列甾体β-碳碱季铵盐衍生物(SCQADs),并首次进行了抗4种革兰氏阳性菌的生物学评价。其中化合物21g对MRSA具有较强的抗菌活性(MIC = 0.5 ~ 1 μg/mL),且具有较低的细胞毒性。同时,衍生物21g能在0.5 h内快速杀死革兰氏阳性菌,且不产生细菌耐药性。初步机理探索表明化合物21g破坏细菌细胞膜发挥抑菌作用。此外,21g在小鼠皮肤脓肿模型中具有较高的体内疗效和较高的存活保护作用。这些结果表明,化合物21g作为一种抗菌剂具有很大的开发潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


