Electric field-enhanced CO2 dissolution and adsorption for geological carbon sequestration in saline aquifers
IF 6.3
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
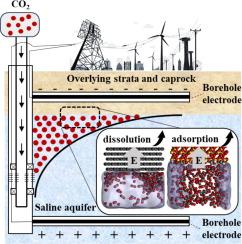
Electric fields can enhance CO2 sequestration in saline aquifers by influencing both dissolution and adsorption, with effects dependent on the field’s orientation, strength, and the rock surface’s wettability. Molecular dynamics simulations reveal that perpendicular electric fields increase CO2 dissolution in water by up to 41.74% on hydrophobic rock surfaces through promoting CO2 desorption, while on hydrophilic rock surfaces, they boost CO2 adsorption by up to 8.27%. These effects intensify with increasing field strength. In contrast, parallel electric fields slightly enhance CO2 adsorption on hydrophobic surfaces but decrease it on hydrophilic ones. The underlying mechanism involves the electric field-induced reorientation of water dipoles, aligning or disrupting hydrogen bonds. In hydrophobic systems, perpendicular fields promote CO2 diffusion into the water phase by inducing perpendicular hydrogen bonds. On hydrophilic surfaces, these fields disrupt hydrogen bonds and non-boned interactions between water and the surface, leading to water detachment and promoting CO2 adsorption. Parallel fields induce parallel hydrogen bonds, impeding CO2 diffusion and reducing its dissolution into the H2O phase (or adsorption onto the rock surface). These findings highlight the potential of electric fields to enhance geologic CO2 sequestration in saline aquifers.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Applied Surface Science
工程技术-材料科学:膜
CiteScore
12.50
自引率
7.50%
发文量
3393
审稿时长
67 days
期刊介绍:
Applied Surface Science covers topics contributing to a better understanding of surfaces, interfaces, nanostructures and their applications. The journal is concerned with scientific research on the atomic and molecular level of material properties determined with specific surface analytical techniques and/or computational methods, as well as the processing of such structures.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


