Applications of pharmacometrics in drug development
IF 17.6
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
The last two decades have witnessed profound changes in how advanced computational tools can help leverage tons of data to improve our knowledge, and ultimately reduce cost and increase productivity in drug development. Pharmacometrics has demonstrated its impact through model-informed drug development (MIDD) approaches. It is now an indispensable component throughout the whole continuum of drug discovery, development, regulatory review, and approval. Today, applications of pharmacometrics are common in designing better trials and accelerating evidence-based decisions. Newly emerging technologies, especially those from data and computer sciences, are being integrated with existing computational tools used in the pharmaceutical industry at a remarkably fast pace. The new challenges faced by the pharmacometrics community are not what or how to contribute, but which optimal MIDD strategy should be adopted to maximize its value in the decision-making process. While we are embracing new innovative approaches and tools, this article discusses how a variety of existing modeling tools, with differentiated advantages and focus, can work in concert to inform drug development.
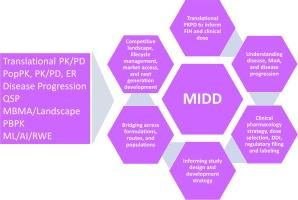

药物计量学在药物开发中的应用
在过去的二十年里,先进的计算工具已经发生了深刻的变化,它们可以帮助利用大量的数据来提高我们的知识,并最终降低成本,提高药物开发的生产率。药物计量学已经通过模型知情药物开发(MIDD)方法证明了它的影响。它现在是整个药物发现、开发、监管审查和批准过程中不可或缺的组成部分。今天,药物计量学的应用在设计更好的试验和加速循证决策方面很常见。新兴技术,特别是来自数据和计算机科学的技术,正以惊人的速度与制药行业使用的现有计算工具相结合。药物计量学界面临的新挑战不是贡献什么或如何贡献,而是应该采用哪种最优的MIDD策略来最大化其在决策过程中的价值。当我们接受新的创新方法和工具时,本文讨论了各种现有的建模工具,具有不同的优势和重点,如何协同工作以通知药物开发。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
28.10
自引率
5.00%
发文量
294
审稿时长
15.1 weeks
期刊介绍:
The aim of the Journal is to provide a forum for the critical analysis of advanced drug and gene delivery systems and their applications in human and veterinary medicine. The Journal has a broad scope, covering the key issues for effective drug and gene delivery, from administration to site-specific delivery.
In general, the Journal publishes review articles in a Theme Issue format. Each Theme Issue provides a comprehensive and critical examination of current and emerging research on the design and development of advanced drug and gene delivery systems and their application to experimental and clinical therapeutics. The goal is to illustrate the pivotal role of a multidisciplinary approach to modern drug delivery, encompassing the application of sound biological and physicochemical principles to the engineering of drug delivery systems to meet the therapeutic need at hand. Importantly the Editorial Team of ADDR asks that the authors effectively window the extensive volume of literature, pick the important contributions and explain their importance, produce a forward looking identification of the challenges facing the field and produce a Conclusions section with expert recommendations to address the issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


