Surface reconstruction of copper(Ⅰ) sulfide electrode regulated electroreduction CO2 selectivity
IF 6.9
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Carbon dioxide (CO2) electroreduction to valuable chemicals is a promising approach for efficient carbon utilization. Herein, we propose an in situ electrochemical reconstruction method to regulate the selectivity of electroreduction of CO2 into CO or HCOOH using a copper sulfide-loaded copper foam (Cu2S/CF) self-supporting electrode. Cu2S/CF under the applied potential of −0.2 V versus reversible hydrogen electrode (vs. RHE) maintain as the intial one, and Cu+ are partially reduced to Cu0 under the applied potential of −0.4 V vs. RHE forming vacancy S defects (VS) in Cu2S. Notably, the Cu2S/CF achieve a potential regulable electroreduction CO2 into CO with faradaic efficiency (FE) of 84.6 % at the applied potential of −0.2 V vs. reversible hydrogen electrode (RHE) and HCOOH with FE of 81.6 % at −0.4 V vs. RHE. Density functional theory (DFT) calculations reveal the different CO2 adsorption configurations of catalytic centers resulting in the difference on the reduction products. Saturation Cu site on Cu2S surface favours to adsorb CO2 with end-on configuration of *COOH for CO production, and unsaturation Cu site on Cu2S-VS surface prefers to the bridge configuration of *O*COH for HCOOH synthesis.

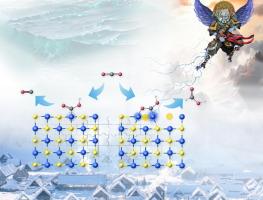
硫化铜(Ⅰ)电极的表面重构调节了电还原CO2的选择性
二氧化碳(CO2)电还原制有价化学品是一种很有前途的高效碳利用方法。在此,我们提出了一种原位电化学重建方法来调节CO2电还原成CO或HCOOH的选择性,该方法使用负载硫化铜的泡沫铜(Cu2S/CF)自支撑电极。Cu2S/CF在−0.2 V对可逆氢电极(VS . RHE)的作用电位下保持初始状态,Cu+在−0.4 V对RHE的作用电位下部分还原为Cu0,在Cu2S中形成空位S缺陷(VS)。值得注意的是,与可逆氢电极(RHE)和HCOOH相比,Cu2S/CF在−0.2 V的电位下实现了电位可调的电还原CO2成CO,其法拉第效率(FE)为84.6 %,在−0.4 V的电位下,其FE为81.6 %。密度泛函理论(DFT)计算揭示了催化中心不同的CO2吸附构型导致还原产物的差异。Cu2S表面的饱和Cu位点有利于以端对*COOH结构吸附CO2生成CO,而Cu2S- vs表面的不饱和Cu位点则有利于以*O*COH桥接结构合成HCOOH。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Applied Surface Science
工程技术-材料科学:膜
CiteScore
12.50
自引率
7.50%
发文量
3393
审稿时长
67 days
期刊介绍:
Applied Surface Science covers topics contributing to a better understanding of surfaces, interfaces, nanostructures and their applications. The journal is concerned with scientific research on the atomic and molecular level of material properties determined with specific surface analytical techniques and/or computational methods, as well as the processing of such structures.
文献相关原料
公司名称
产品信息
阿拉丁
Cetyltrimethylammonium bromide (CTAB)
阿拉丁
Cetyltrimethylammonium bromide
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


