Protecting Group Control of Hydroxyketone-Hemiketal Tautomeric Equilibrium Enables the Stereoselective Synthesis of a 1′-Azido C-Nucleoside
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
Journal of Organic Chemistry
Pub Date : 2024-11-19
DOI:10.1021/acs.joc.4c0198110.1021/acs.joc.4c01981
引用次数: 0
Abstract
The synthesis of 1′-azido C-nucleosides is described to expand the set of azide-functionalized nucleosides for bioorthogonal applications and as potential antiviral drugs. Lewis acid-promoted azidation of a nucleoside hemiketal resulted in the formation of a tetrazole through a Schmidt reaction manifold. Conformational control to prevent ring–chain tautomerism enabled efficient 1′-azidation with complete β-diastereoselectivity. The unique reactivity and further derivation of the 1′-azido C-nucleosides are also reported.
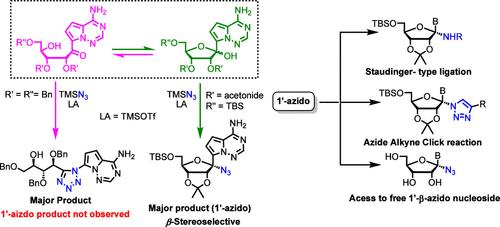
羟基酮-半晶互变异构平衡的保护基团控制使1 ' -叠氮基c -核苷的立体选择性合成成为可能
本研究介绍了 1′-叠氮 C 核苷的合成,从而扩大了叠氮功能化核苷的范围,使其可用于生物正交应用并成为潜在的抗病毒药物。路易斯酸促进核苷半金属的叠氮化,通过施密特反应歧管形成四氮唑。为防止环链同分异构而进行的构象控制实现了具有完全 β-非对映选择性的高效 1′-唑化反应。报告还介绍了 1′-叠氮 C 核苷的独特反应性和进一步衍生。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


