Utilizing Engineered Bacteria as “Cell Factories” In Vivo for Intracellular RNA-Loaded Outer Membrane Vesicles’ Self-Assembly in Tumor Treatment
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
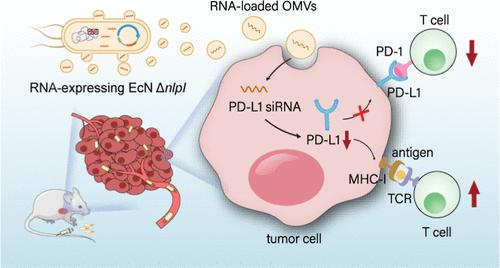
Delivery systems play a crucial role in RNA therapy. However, the current RNA delivery system involves complex preparation and transport processes, requiring RNA preassembly in vitro, transportation at low temperatures throughout, and possibly multiple injections for improved therapeutic efficacy. To address these challenges, we developed a simple and efficient RNA delivery system. This system only requires the injection of engineered bacteria, which serve as in vivo “cell factories” for continuous production of the target RNA. The RNA can self-assemble with engineered bacteria’s outer membrane vesicles (OMVs), facilitating in vivo RNA delivery. Experimental results demonstrated that this system allowed effective delivery with excellent stability and continuity for various types of RNA, including mRNA, miRNA, and siRNA. And the relative abundance of target RNA in the OMVs was 104–107 times higher than that in the mock group. We took the delivery of PD-L1 siRNA for tumor treatment as an example and found that this system could effectively downregulate the gene expression of PD-L1 by approximately twofold. Notably, a single injection of engineered bacteria achieved a significant tumor suppression of 49.37% in vivo. This research provides promising insights into the RNA delivery system for tumor therapy.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


