Solvent-Driven Na Storage in SnS2 Anodes: Atomistic Simulation-Guided Strategies for Reversible Reactions, Solid Electrolyte Interphase, and Morphological Transformation
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
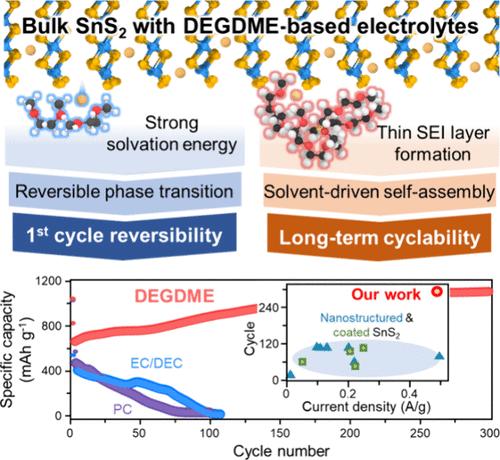
Crystalline SnS2 accommodates Na ions through intercalation–conversion–alloying (ICA) reactions, exhibiting a natural potential for high energy storage, while its layered structure facilitates rapid charging. However, these intrinsic advantages are not fully realized in practical battery applications. Herein, utilizing an innovative integration of machine-learning-based thermodynamics, artificial-neural-network-assisted molecular dynamics, and density functional theory, specific solvents are demonstrated to effectively tailor the reaction pathways. This strategy not only steers phase transition pathways but also significantly reduces the formation of the solid electrolyte interphase (SEI), which is a common issue in recent battery research. These characteristics of solvents enable reversible ICA reactions and also aid the transformation of microsized SnS2 particles into 3D porous nanostructures with minimal SEI formation. The performance of our Na–SnS2 half-cells achieve 1100 mAh g–1 (97% of the theoretical capacity) at 0.5 C, placing them among the top performers for Na storage. By moving beyond the traditional view of electrolyte solvents as a simple medium for ion transport, this work highlights the critical impact of solvent selection on enabling reversible reactions and morphological transformation of SnS2 anodes with minimal SEI formation and setting benchmarks for anode performance in energy storage systems based on ICA reactions.
SnS2 阳极中溶剂驱动的 Na 储存:原子模拟引导的可逆反应、固体电解质相间和形态转变策略
晶体SnS2通过嵌入-转换-合金化(ICA)反应容纳Na离子,表现出高能量存储的天然潜力,而其层状结构有利于快速充电。然而,这些内在的优势在实际的电池应用中并没有完全实现。本文利用基于机器学习的热力学、人工神经网络辅助的分子动力学和密度泛函理论的创新集成,证明了特定溶剂可以有效地定制反应途径。这种策略不仅控制了相变途径,而且显著减少了固体电解质间相(SEI)的形成,这是最近电池研究中的一个常见问题。溶剂的这些特性使得可逆的ICA反应成为可能,也有助于将微尺寸的SnS2颗粒转化为具有最小SEI形成的3D多孔纳米结构。我们的Na - sns2半电池在0.5℃下的性能达到1100 mAh g-1(97%的理论容量),使其成为Na存储性能最好的电池之一。通过超越电解液溶剂作为离子传输的简单介质的传统观点,本工作强调了溶剂选择对实现可逆反应和SnS2阳极形态转变的关键影响,并以最小的SEI形成为基础,为基于ICA反应的储能系统中的阳极性能设定了基准。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


