Engineering Mild-Photothermal Responsive and NO Donor Prussian Blue Nanozymes Using Mild Synthesis for Inflammation Regulation and Bacterial Eradication in Periodontal Disease
IF 27.4
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
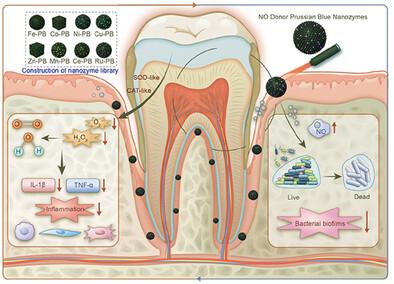
Periodontitis, an infectious disease of periodontal tissues caused by oral bacterial biofilms, is characterized by reactive oxygen species (ROS) accumulation and immune microenvironment imbalance. Multifunctional nanozymes, leveraging their physiochemical properties and enzymatic activities, offer promising antibacterial and anti-inflammatory strategies for managing periodontitis. In particular, Prussian blue nanozymes (PBzymes) exhibit exceptional ROS control due to their robust catalytic activity, diverse antioxidant functions, and high biocompatibility. However, the practical application of traditional high-temperature synthesis methods is limited. This study introduces a class of metal-engineered PBzymes synthesized at room temperature, identified for their potent antioxidative activity and excellent photothermal performance at mild temperatures. Nitric oxide (NO) gas therapy offers promising strategies for targeting deep infections in periodontal tissues. Thus, sodium nitroprusside is introduced into PBzyme to create SPBzyme via an in situ loading method. NO release by SPBzyme enhances antibacterial effects and overcomes resistance linked to bacterial biofilms, resulting in mild-photothermal antibacterial properties and synergistic antioxidant effects. In vitro antibacterial assays demonstrate the superior efficacy of SPBzyme under mild temperature conditions and near-infrared light exposure. Furthermore, SPBzyme effectively reduces inflammation and has positive therapeutic effects in periodontal animal models. Overall, mild-temperature photothermal NO release nanozyme therapy represents a novel approach for treating periodontitis.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Materials
工程技术-材料科学:综合
CiteScore
43.00
自引率
4.10%
发文量
2182
审稿时长
2 months
期刊介绍:
Advanced Materials, one of the world's most prestigious journals and the foundation of the Advanced portfolio, is the home of choice for best-in-class materials science for more than 30 years. Following this fast-growing and interdisciplinary field, we are considering and publishing the most important discoveries on any and all materials from materials scientists, chemists, physicists, engineers as well as health and life scientists and bringing you the latest results and trends in modern materials-related research every week.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


