Water Electrooxidation Kinetics Clarified by Time-Resolved X-Ray Absorption and Electrochemical Impedance Spectroscopy for a Bulk-Active Cobalt Material
IF 24.4
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
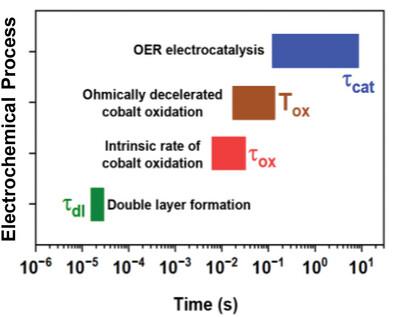
Water oxidation, the oxygen evolution reaction (OER), is the anodic process in electrocatalytic production of hydrogen and further green fuels. Transition-metal oxyhydroxides with bulk-phase OER activity of the complete material or amorphized near-surface regions are of prime application interest, but their basic electrochemical properties are insufficiently understood. Here the timescale of functional processes is clarified by time-resolved X-ray absorption spectroscopy and electrochemical impedance spectroscopy (EIS) for a thickness-series of cobalt oxyhydroxides films (about 35–550 nm). At the outer material surface, an electric double-layer is formed in microseconds followed by clearly cobalt-centered redox-state changes of the bulk material in the low millisecond domain and a slow chemical step of O2-formation, within hundreds of milliseconds. Conceptually interesting, the electrode potential likely controls the OER rate indirectly by driving the catalyst material to an increasingly oxidized state which promotes the rate-limiting chemical step. Rate constants are derived for redox chemistry and catalysis from EIS data of low-thickness catalyst films; at higher thicknesses, catalyst-internal charge transport limitations become increasingly relevant. Relations between electrochemically active surface area, double-layer capacitance, and redox (pseudo-)capacitance are discussed. These results can increase the power of EIS analyses and support knowledge-guided optimization of a broader class of OER catalyst materials.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Energy Materials
CHEMISTRY, PHYSICAL-ENERGY & FUELS
CiteScore
41.90
自引率
4.00%
发文量
889
审稿时长
1.4 months
期刊介绍:
Established in 2011, Advanced Energy Materials is an international, interdisciplinary, English-language journal that focuses on materials used in energy harvesting, conversion, and storage. It is regarded as a top-quality journal alongside Advanced Materials, Advanced Functional Materials, and Small.
With a 2022 Impact Factor of 27.8, Advanced Energy Materials is considered a prime source for the best energy-related research. The journal covers a wide range of topics in energy-related research, including organic and inorganic photovoltaics, batteries and supercapacitors, fuel cells, hydrogen generation and storage, thermoelectrics, water splitting and photocatalysis, solar fuels and thermosolar power, magnetocalorics, and piezoelectronics.
The readership of Advanced Energy Materials includes materials scientists, chemists, physicists, and engineers in both academia and industry. The journal is indexed in various databases and collections, such as Advanced Technologies & Aerospace Database, FIZ Karlsruhe, INSPEC (IET), Science Citation Index Expanded, Technology Collection, and Web of Science, among others.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


