Cationic Coordination Modification Drives Birefringence and Nonlinear Effect Double Lifting in Sulfate
IF 4.3
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
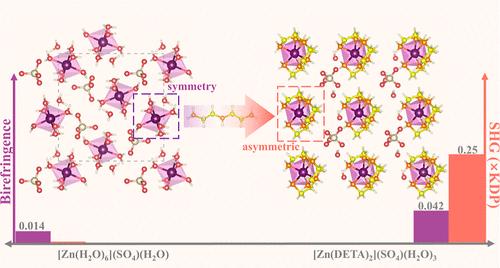
As nonlinear optical (NLO) crystals, sulfates have the superiority of transparency for ultraviolet (UV) light, but they are often troubled by small nonlinear coefficients and birefringence owing to the high symmetry of the [SO4]2– group. By introducing two neutral diethylenetriamine (DETA) molecules to replace the six coordinated water molecules of the [Zn(H2O)6]2+ complex cation in [Zn(H2O)6](SO4)(H2O), a new sulfate with an acentric structure, namely, [Zn(DETA)2](SO4)(H2O)3, has been designed and synthesized. Structural investigation reveals that the coordination modification of Zn2+ ion tremendously enhances its intraoctahedral distortion. The formed distorted [Zn(DETA)2]2+ cations and the [SO4]2– groups feature an optimized arrangement, endowing [Zn(DETA)2](SO4)(H2O)3 with enhancements in both second harmonic generation (SHG) intensity, from undetectable to 0.25 × KH2PO4 (KDP), and birefringence, from 0.014 to 0.042 at 1064 nm. Despite the slight compromise made in the light transmission range, [Zn(DETA)2](SO4)(H2O)3 still possesses a short absorption edge of 220 nm, retaining the majority of the light transmission range in the solar-blind region. Our work provides a novel and applicable approach of cationic coordination modification to improve the nonlinear optical coefficient and birefringence of sulfates.
阳离子配位改性驱动硫酸盐双折射和非线性双提升效应
作为非线性光学(NLO)晶体,硫酸盐具有对紫外线(UV)透明的优越性,但由于[SO4]2-基团的高度对称性,它们往往受到非线性系数小和双折射的困扰。通过引入两个中性二乙烯三胺(DETA)分子取代[Zn(H2O)6](SO4)(H2O)中[Zn(H2O)6]2+阳离子络合物的六个配位水分子,设计并合成了一种具有心形结构的新型硫酸盐,即[Zn(DETA)2](SO4)(H2O)3。结构研究表明,Zn2+ 离子的配位修饰极大地增强了其八面体内畸变。所形成的畸变[Zn(DETA)2]2+阳离子和[SO4]2-基团具有优化的排列,使[Zn(DETA)2](SO4)(H2O)3的二次谐波发生(SHG)强度从检测不到提高到 0.25 × KH2PO4 (KDP),在 1064 纳米波长处的双折射从 0.014 提高到 0.042。尽管在透光范围上略有妥协,[Zn(DETA)2](SO4)(H2O)3 仍具有 220 纳米的短吸收边,保留了日盲区的大部分透光范围。我们的研究为改善硫酸盐的非线性光学系数和双折射提供了一种新颖而适用的阳离子配位修饰方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
文献相关原料
公司名称
产品信息
阿拉丁
ZnSO4·7H2O
阿拉丁
ZnSO4·7H2O
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


