Effects of EDTA and Bicarbonate on U(VI) Reduction by Reduced Nontronite
IF 10.8
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
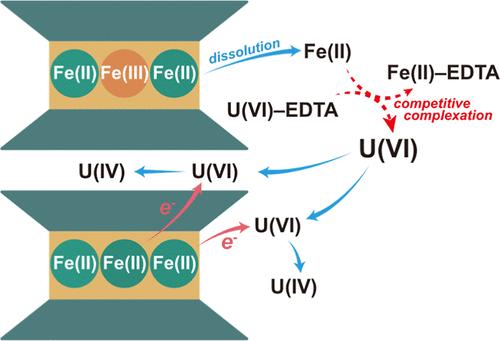
Widespread Fe-bearing clay minerals are potential materials capable of reducing and immobilizing U(VI). However, the kinetics of this process and the impact of environmental factors remain unclear. Herein, we investigated U(VI) reduction by chemically reduced nontronite (rNAu-2) in the presence of EDTA and bicarbonate. U(VI) was completely reduced within 192 h by rNAu-2 alone, and higher Fe(II) in rNAu-2 resulted in a higher U(VI) reduction rate. However, the presence of EDTA and NaHCO3 initially inhibited U(VI) reduction by forming stable U(VI)–EDTA/carbonato complexes and thus preventing U(VI) from adsorbing onto the rNAu-2 surface. However, over time, EDTA facilitated the dissolution of rNAu-2, releasing Fe(II) into solution. Released Fe(II) competed with U(VI) to form Fe(II)–EDTA complexes, thus freeing U(VI) from negatively charged U(VI)–EDTA complexes to form positively charged U(VI)–OH complexes, which ultimately promoted U(VI) adsorption and triggered its reduction. In the NaHCO3 system, U(VI) complexed with carbonate to form U(VI)–carbonato complexes, which partially inhibited adsorption to the rNAu-2 surface and subsequent reduction. The reduced U(IV) largely formed uraninite nanoparticles, with a fraction present in the rNAu-2 interlayer. Our results demonstrate the important impacts of clay minerals, organic matter, and bicarbonate on U(VI) reduction, providing crucial insights into the uranium biogeochemistry in the subsurface environment and remediation strategies for uranium-contaminated environments.
乙二胺四乙酸和碳酸氢盐对还原壬铁矿还原铀(VI)的影响
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


