Capture of Polysulfides Enabled by a Nitrogen-Doped Carbon-Coated Halloysite Nanotube-Modified Separator to Enhance Performance for Lithium–Sulfur Batteries
IF 8.3
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
Lithium–sulfur (Li–S) batteries stand out as highly promising energy storage systems because of their superior theoretical capacity and the affordability of sulfur as an active material. However, their inherent drawbacks have hindered the commercialization of Li–S batteries. Of these, the polysulfide shuttle effect is one of the most critical issues, leading to the rapid decline in battery capacity. To specifically address this issue, we successfully synthesized nitrogen-doped carbon-coated halloysite nanotubes (HNT@NC) using a one-step sintering method and modified the Celgard 2325 separator on the side facing the sulfur cathode (HNT@NC-Separator). The study found that HNT@NC-Separator exhibits excellent electrolyte wettability and superb mechanical strength. Its surface has abundant polar sites that effectively capture lithium polysulfides, thereby improving the cycling and rate performance of Li–S batteries. At a current density of 0.2 C, the Li–S battery assembled with the HNT@NC-Separator achieved an initial discharge capacity of 840.8 mAh g–1, maintaining a capacity of 486.1 mAh g–1 after 100 cycles. At a current density of 1 C, the initial discharge capacity was 770.4 mAh g–1, maintaining a capacity of 412.9 mAh g–1 after 100 cycles. In the rate performance test, the capacity retention rate exceeded 75%.
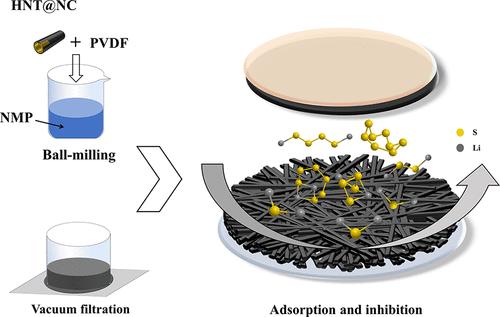
用掺氮碳包覆的霍洛石纳米管改性分离器捕获多硫化物以提高锂硫电池的性能
锂硫(Li-S)电池是一种极具潜力的储能系统,因为它具有超强的理论容量,而且作为活性材料的硫价格低廉。然而,其固有的缺点阻碍了锂硫电池的商业化。其中,多硫化物穿梭效应是最关键的问题之一,它导致电池容量迅速下降。为了有针对性地解决这一问题,我们采用一步烧结法成功合成了掺氮碳包覆的埃洛石纳米管(HNT@NC),并在面向硫阴极的一侧对 Celgard 2325 隔膜进行了改性(HNT@NC-隔膜)。研究发现,HNT@NC-Separator 具有优异的电解质润湿性和超强的机械强度。其表面具有丰富的极性位点,可有效捕获多硫化锂,从而改善锂-S 电池的循环和速率性能。在 0.2 C 的电流密度下,使用 HNT@NC 隔离层组装的锂-S 电池的初始放电容量为 840.8 mAh g-1,循环 100 次后容量保持在 486.1 mAh g-1。在电流密度为 1 C 时,初始放电容量为 770.4 mAh g-1,100 次循环后容量保持在 412.9 mAh g-1。在速率性能测试中,容量保持率超过 75%。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


