An experimental review of the reaction paths followed by alkali-activated slag pastes
IF 10.9
1区 工程技术
Q1 CONSTRUCTION & BUILDING TECHNOLOGY
引用次数: 0
Abstract
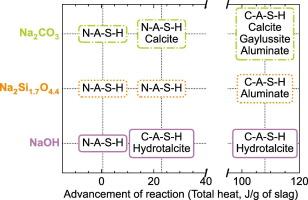
NMR, XRD, mechanical strength measurements, bound water quantification and isothermal calorimetry were combined to contrast the degree of reaction and the paths followed by slag under the influence of three activators: NaOH, Na2CO3, and Na2Si1.7O4.4. NaOH-activation led to the concomitant formation of a very ordered C-A-S-H gel and of hydrotalcite, giving rise to early mechanical strength. For Na2Si1.7O4.4, a N-A-S-H gel formed first due to the high quantities of silicon in solution. This led to quick setting but no mechanical strength. Later, an amorphous C-A-S-H gel provided mechanical strength, while an aluminate phase precipitated. Finally, Na2CO3-activation also led to the formation of N-A-S-H, formation favored by the initial consumption of calcium to form calcite. This did not bring any real structuration and mechanical strength. Only after a few days of hydration did the mechanical strength improve with the precipitation of amorphous C-A-S-H, an ill-defined hydrated aluminate phase, and of gaylussite.
碱活性渣浆反应路径的实验回顾
结合核磁共振、X 射线衍射、机械强度测量、结合水定量和等温量热法,对比了炉渣在三种活化剂影响下的反应程度和路径:NaOH、Na2CO3 和 Na2Si1.7O4.4。NaOH 活化会同时形成非常有序的 C-A-S-H 凝胶和水滑石,从而产生早期机械强度。对于 Na2Si1.7O4.4,由于溶液中含有大量硅,首先形成了 N-A-S-H 凝胶。这导致了快速凝固,但没有机械强度。之后,无定形的 C-A-S-H 凝胶提供了机械强度,同时铝酸盐相析出。最后,Na2CO3 的活化也导致了 N-A-S-H 的形成,最初的钙消耗有利于形成方解石。但这并没有带来真正的结构和机械强度。只有在水化几天后,随着无定形的 C-A-S-H、不明确的水合铝酸盐相和基铝酸盐的析出,机械强度才有所提高。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cement and Concrete Research
工程技术-材料科学:综合
CiteScore
20.90
自引率
12.30%
发文量
318
审稿时长
53 days
期刊介绍:
Cement and Concrete Research is dedicated to publishing top-notch research on the materials science and engineering of cement, cement composites, mortars, concrete, and related materials incorporating cement or other mineral binders. The journal prioritizes reporting significant findings in research on the properties and performance of cementitious materials. It also covers novel experimental techniques, the latest analytical and modeling methods, examination and diagnosis of actual cement and concrete structures, and the exploration of potential improvements in materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


