Effects of Cosolvent and Nonsolvating Solvent on the Structural Dynamics of Organic Electrolytes in Sodium-Ion Batteries
IF 8.3
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
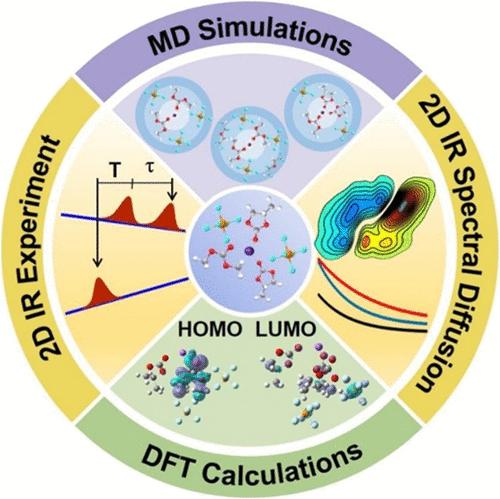
In sodium-ion batteries (SIBs), the performance of a single solvent often does not meet actual requirements and a cosolvent or nonsolvating solvent is needed. However, the effect of these electrolyte additives on the solvation structure and dynamics of Na+ in SIBs is yet to be fully understood. Herein, electrolyte structural dynamics are examined for NaPF6 in dimethyl carbonate (DMC) with 1,1,2,2-tetrafluoro-2,2,2-trifluoroethoxy ethane (HFE) as the nonsolvating solvent or propylene carbonate (PC) as the cosolvent using steady-state and time-resolved infrared (IR) spectroscopies. Molecular dynamics simulations show that the solvation size of Na+ decreases with a loosened structure upon adding the nonsolvating solvent, whereas its first solvation shell becomes denser and the second one becomes softened with decreased participation of the PF6– anion upon adding the cosolvent. While a decreased participation of DMC in the solvation layer of Na+ is suggested by linear IR results, an increased structural inhomogeneity (and hence overall more dynamical fluctuations) is found for Na+-coordinated DMC upon adding both additives by two-dimensional (2D) IR spectroscopy where the carbonyl stretch is used as a probe. The Na+/DMC/PC complex is found to structurally evolve slower than both Na+/DMC and Na+/DMC/HFE complexes on the picosecond time scales by spectral diffusion dynamics extracted from 2D IR diagonal signals. Frontier orbital theory calculations also indicate that both solvent additives are beneficial to increasing the stability of PF6–. The results obtained in this work provide important insights into the roles played by the solvent additives in influencing the solvation structures and dynamics of Na+, which are critical for understanding the Na+ transfer mechanism in SIBs.
助溶剂和非溶剂溶剂对钠离子电池有机电解质结构动力学的影响
在钠离子电池(SIB)中,单一溶剂的性能往往无法满足实际要求,因此需要共溶剂或非溶解溶剂。然而,这些电解质添加剂对钠离子电池中 Na+ 的溶解结构和动力学的影响尚有待充分了解。在此,我们利用稳态和时间分辨红外(IR)光谱研究了以 1,1,2,2-四氟-2,2,2-三氟乙氧基乙烷(HFE)为非溶剂或以碳酸丙烯酯(PC)为共溶剂的碳酸二甲酯(DMC)中 NaPF6 的电解质结构动力学。分子动力学模拟表明,加入非溶解溶剂后,Na+ 的溶解度减小,结构变得松散;而加入共溶剂后,随着 PF6- 阴离子参与度的降低,Na+ 的第一个溶解壳变得致密,第二个溶解壳变得柔软。线性红外结果表明,DMC 在 Na+ 溶胶层中的参与程度降低,但通过二维(2D)红外光谱(以羰基伸展为探针)发现,添加两种添加剂后,Na+ 配位的 DMC 的结构不均匀性增加(因此整体动态波动更大)。从二维红外对角线信号中提取的光谱扩散动力学发现,在皮秒时间尺度上,Na+/DMC/PC 复合物的结构演变速度比 Na+/DMC 和 Na+/DMC/HFE 复合物都要慢。前沿轨道理论计算也表明,这两种溶剂添加剂都有利于提高 PF6- 的稳定性。这项工作中获得的结果为了解溶剂添加剂在影响 Na+ 的溶解结构和动力学方面所起的作用提供了重要的启示,而这对于理解 SIB 中 Na+ 的转移机制至关重要。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
文献相关原料
公司名称
产品信息
阿拉丁
Dimethyl carbonate (DMC)
阿拉丁
Propylene carbonate (PC)
阿拉丁
Dimethyl carbonate (DMC)
阿拉丁
Propylene carbonate (PC)
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


