Effects of Cosolvent and Nonsolvating Solvent on the Structural Dynamics of Organic Electrolytes in Sodium-Ion Batteries
IF 8.3
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
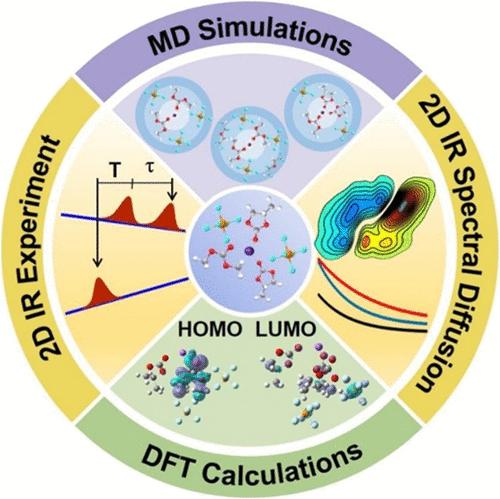
In sodium-ion batteries (SIBs), the performance of a single solvent often does not meet actual requirements and a cosolvent or nonsolvating solvent is needed. However, the effect of these electrolyte additives on the solvation structure and dynamics of Na+ in SIBs is yet to be fully understood. Herein, electrolyte structural dynamics are examined for NaPF6 in dimethyl carbonate (DMC) with 1,1,2,2-tetrafluoro-2,2,2-trifluoroethoxy ethane (HFE) as the nonsolvating solvent or propylene carbonate (PC) as the cosolvent using steady-state and time-resolved infrared (IR) spectroscopies. Molecular dynamics simulations show that the solvation size of Na+ decreases with a loosened structure upon adding the nonsolvating solvent, whereas its first solvation shell becomes denser and the second one becomes softened with decreased participation of the PF6– anion upon adding the cosolvent. While a decreased participation of DMC in the solvation layer of Na+ is suggested by linear IR results, an increased structural inhomogeneity (and hence overall more dynamical fluctuations) is found for Na+-coordinated DMC upon adding both additives by two-dimensional (2D) IR spectroscopy where the carbonyl stretch is used as a probe. The Na+/DMC/PC complex is found to structurally evolve slower than both Na+/DMC and Na+/DMC/HFE complexes on the picosecond time scales by spectral diffusion dynamics extracted from 2D IR diagonal signals. Frontier orbital theory calculations also indicate that both solvent additives are beneficial to increasing the stability of PF6–. The results obtained in this work provide important insights into the roles played by the solvent additives in influencing the solvation structures and dynamics of Na+, which are critical for understanding the Na+ transfer mechanism in SIBs.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


