Reactor design of oxidative species generation for process intensification of photolytic ozonation
IF 4.1
2区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
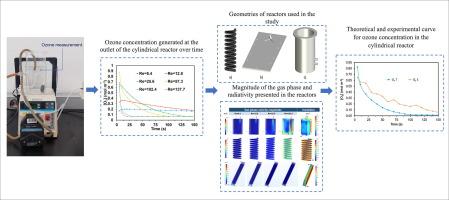
The experimental and theoretical productions of oxygen, ozone, and hydrogen peroxide are accounted for during a photolytic ozonation (PO) process, with the aim of selecting a suitable reactor maximizing the interaction between ozone and radiation. In this process, ozone (O3) reacts with water (H2O) to produce oxygen (O2) and hydrogen peroxide (H2O2). Additionally, ozone reacts with hydrogen peroxide, leading to the formation of the ozonide radical anion (O3•-), oxygen (O2), and protons (H+). An ozonide radical trapping test was conducted using Sulfamethoxazole (SMX) as a model contaminant. To analyze the presence of ozonide, ascorbic acid was supplied to the cylindrical reactor. The oxidation processes for radical detection lasted for 90 min. The inhibition of SMX oxidation confirmed the presence of the ozonide radical. Experimental measurements are firstly evaluated to estimate kinetic constants using in-line sensors and permanganometry method, which are subsequently connected in a proposed reaction model applied to three different reactor geometries (e.g. parallel plates, serpentine, cylindrical). Species distribution is considered in the fluid dynamics of these configurations in terms of water inlet velocity at Reynolds numbers of 6.4, 12.8, 25.6, and 102.4. It is found that experimental data for oxidant productions are appropriately fitted by theoretical models, confirming the validity of the proposed model kinetic constants. Likewise, the kinetic reaction model can be simplified into two main reactions, with the major products being H2O2, O2, and O3•-. While ozone concentrations increase at higher Reynolds numbers, hydrogen peroxide and oxygen exhibited linear growth over time, and O3•- production showed nonlinear behavior. The cylindrical reactor design demonstrates optimal reaction efficiency, combining effective mixing and continuous operation at significant cost savings due to reduced energy requirements, making it suitable for applications involving oxidant production using radiation and ozone. To this concern, the significance of O3•- generation, flow regime and reactor geometry are determining factors to maximize the oxidant concentrations.

光解臭氧化过程强化氧化物质生成反应器设计
对光解臭氧(PO)过程中氧气、臭氧和过氧化氢的实验和理论生成量进行了核算,目的是选择一个合适的反应器,最大限度地发挥臭氧和辐射之间的相互作用。在此过程中,臭氧(O3)与水(H2O)反应生成氧气(O2)和过氧化氢(H2O2)。此外,臭氧还会与过氧化氢发生反应,形成臭氧自由基阴离子(O3--)、氧气(O2)和质子(H+)。以磺胺甲噁唑(SMX)为污染物模型,进行了臭氧自由基捕获试验。为了分析臭氧的存在,向圆柱形反应器中加入了抗坏血酸。检测自由基的氧化过程持续了 90 分钟。对 SMX 氧化的抑制证实了臭氧自由基的存在。首先对实验测量结果进行评估,利用在线传感器和高锰计方法估算动力学常数,然后将其与应用于三种不同反应器几何形状(如平行板、蛇形、圆柱形)的拟议反应模型相联系。在雷诺数为 6.4、12.8、25.6 和 102.4 时,根据进水速度考虑了这些配置的流体动力学中的物种分布。研究发现,氧化剂生成的实验数据与理论模型相吻合,这证实了所提出的模型动力学常数的有效性。同样,动力学反应模型可简化为两个主要反应,主要产物为 H2O2、O2 和 O3--。虽然臭氧浓度在雷诺数越高时越高,但过氧化氢和氧气随着时间的推移呈线性增长,而 O3--的生成则呈非线性行为。圆柱形反应器的设计展示了最佳的反应效率,将有效混合和连续运行结合在一起,同时由于降低了能源需求而大大节约了成本,使其适用于使用辐射和臭氧生产氧化剂的应用。在这方面,O3 生成的重要性、流动机制和反应器的几何形状是使氧化剂浓度最大化的决定性因素。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Science
工程技术-工程:化工
CiteScore
7.50
自引率
8.50%
发文量
1025
审稿时长
50 days
期刊介绍:
Chemical engineering enables the transformation of natural resources and energy into useful products for society. It draws on and applies natural sciences, mathematics and economics, and has developed fundamental engineering science that underpins the discipline.
Chemical Engineering Science (CES) has been publishing papers on the fundamentals of chemical engineering since 1951. CES is the platform where the most significant advances in the discipline have ever since been published. Chemical Engineering Science has accompanied and sustained chemical engineering through its development into the vibrant and broad scientific discipline it is today.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


