In Situ Metal Vacancy Filling in Stable Pd-Sn Intermetallic Catalyst for Enhanced C?C Bond Cleavage in Ethanol Oxidation
IF 27.4
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
A common challenge in electrochemical processes is developing high performance, stable catalysts for specific chemical reactions. In this work, a Pd-Sn intermetallic compound with Pd site deficiency (Pd1.9−xSn) (x = 0.06) and trace amount of SnOx was synthesised by controlled process. Under the electrochemical conditions, the deficient Pd site is filled by metallic Sn, which generates a highly active and stable (Pd1.84Sn0.06)Sn catalyst for ethanol oxidation reaction (EOR). The crystal structure and atomic arrangements for synthesized and in situ generated compound are comprehensively characterized by various spectroscopic techniques. The in situ generated catalyst exhibits excellent performance toward EOR (anodic reaction in fuel cell), which outperforms the state-of-the-art Pd/C catalyst by three times in terms of activity. Furthermore, it is observed that the catalyst preferentially cleaves the CC bond in ethanol, which is a crucial process that enhances the efficiency of the fuel cells. The catalyst retains its superlative activity even after 1500 cycles of continuous operation. The mechanism for EOR and CC bond cleavage is evidenced by operando Infra Red spectroscopy and Differential Electrochemical Mass Spectroscopy (DEMS), and the driving force toward excellent performance has been proposed via theoretical calculations.
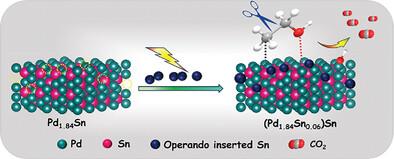
电化学过程中的一个共同挑战是为特定化学反应开发高性能、稳定的催化剂。在这项工作中,通过受控工艺合成了一种钯-锡金属间化合物,该化合物具有钯位缺陷(Pd1.9-xSn)(x = 0.06)和微量氧化锡。在电化学条件下,缺失的 Pd 位点被金属 Sn 填满,从而生成了高活性、高稳定性的 (Pd1.84Sn0.06)Sn 催化剂,用于乙醇氧化反应(EOR)。通过各种光谱技术对合成化合物和原位生成化合物的晶体结构和原子排列进行了全面表征。原位生成的催化剂在乙醇氧化反应(燃料电池中的阳极反应)中表现出优异的性能,其活性比最先进的 Pd/C 催化剂高出三倍。此外,还观察到催化剂优先裂解乙醇中的 CC 键,这是提高燃料电池效率的关键过程。该催化剂即使在连续运行 1500 个循环后仍能保持极高的活性。操作红外光谱和差分电化学质谱(DEMS)证明了 EOR 和 CC 键裂解的机理,并通过理论计算提出了实现卓越性能的驱动力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Materials
工程技术-材料科学:综合
CiteScore
43.00
自引率
4.10%
发文量
2182
审稿时长
2 months
期刊介绍:
Advanced Materials, one of the world's most prestigious journals and the foundation of the Advanced portfolio, is the home of choice for best-in-class materials science for more than 30 years. Following this fast-growing and interdisciplinary field, we are considering and publishing the most important discoveries on any and all materials from materials scientists, chemists, physicists, engineers as well as health and life scientists and bringing you the latest results and trends in modern materials-related research every week.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


