Synthesis of polymer-clindamycin conjugates through lipase-catalyzed esterification and RAFT polymerization
IF 4.1
2区 化学
Q2 POLYMER SCIENCE
引用次数: 0
Abstract
Relative to free antibiotics, polymer-antibiotic conjugates (PACs) can possess modified solubility, sustained release behavior, and prolonged bioactivity in biological systems. As one of the most potent and ubiquitous antibiotics, clindamycin (Clin) has broad-spectrum antibiotic activity with versatile medical applications. However, polymer-Clin conjugates have not been reported yet. This can be partly ascribed to the difficulties in selective modification of Clin which possesses multiple reactive hydroxyl groups. In this study, we employed immobilized lipase as a bio-based catalyst for the facile and highly regioselective synthesis of a methacrylic-functionalized monomer-Clin conjugate via a one-step reaction. Reversible addition fragmentation chain transfer (RAFT) polymerization was then employed to synthesize copolymers of the monomer-Clin conjugate with 2-hydroxymethyl methacrylate or 3-[(3-acrylamidopropyl) dimethylammonio]propanoate to achieve water-insoluble and water-soluble Clin-containing PACs, respectively. These PACs possessed well-defined structures with high Clin content (33–46 wt%), as confirmed by 1H NMR and gel permeation chromatography characterizations. Living nature of the RAFT process for the synthesis of PACs was verified by a chain-extension experiment. With sustained Clin release behavior, these PACs further demonstrated notable antibacterial activities against Streptococcus mutans, as verified by zone of inhibition tests. Collectively, this work presents an efficient method to synthesize different types of Clin-containing PACs, with potential for use in diverse antibacterial applications.
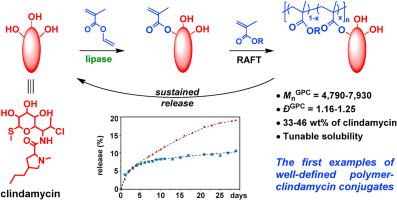

脂肪酶催化酯化和RAFT聚合合成聚合物-克林霉素偶联物
与游离抗生素相比,聚合物-抗生素偶联物(pac)在生物系统中具有改良的溶解度、缓释行为和延长的生物活性。克林霉素(clindamycin, Clin)具有广谱抗菌活性,具有广泛的医学用途,是目前最有效、最普遍的抗生素之一。然而,聚合物-克林偶联物尚未见报道。这可能部分归因于具有多个活性羟基的Clin在选择性修饰方面的困难。在这项研究中,我们采用固定化脂肪酶作为生物基催化剂,通过一步反应简便地合成了甲基丙烯酸功能化单体- clin共轭物。然后采用可逆加成断裂链转移(RAFT)聚合,合成了单体- clin与2-羟甲基丙烯酸甲酯或3-[(3-丙烯酰胺丙基)二甲酰胺]丙酸酯的共聚物,分别得到了不水溶性和水溶性的含clin PACs。经1H NMR和凝胶渗透色谱表征证实,这些pac具有明确的结构,具有高clint含量(33-46 wt%)。通过扩链实验验证了RAFT工艺合成PACs的活性。这些pac具有持续的Clin释放行为,进一步显示出对变形链球菌的显著抗菌活性,抑制区试验证实了这一点。总的来说,这项工作提出了一种有效的方法来合成不同类型的含clinc的pac,具有在各种抗菌应用中的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Polymer
化学-高分子科学
CiteScore
7.90
自引率
8.70%
发文量
959
审稿时长
32 days
期刊介绍:
Polymer is an interdisciplinary journal dedicated to publishing innovative and significant advances in Polymer Physics, Chemistry and Technology. We welcome submissions on polymer hybrids, nanocomposites, characterisation and self-assembly. Polymer also publishes work on the technological application of polymers in energy and optoelectronics.
The main scope is covered but not limited to the following core areas:
Polymer Materials
Nanocomposites and hybrid nanomaterials
Polymer blends, films, fibres, networks and porous materials
Physical Characterization
Characterisation, modelling and simulation* of molecular and materials properties in bulk, solution, and thin films
Polymer Engineering
Advanced multiscale processing methods
Polymer Synthesis, Modification and Self-assembly
Including designer polymer architectures, mechanisms and kinetics, and supramolecular polymerization
Technological Applications
Polymers for energy generation and storage
Polymer membranes for separation technology
Polymers for opto- and microelectronics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


