CuPd/Al2O3 single atom alloy catalyst facilitates the catalytic transfer hydrodeoxygenation of furfural to 2-methylfuran
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Alloying Cu-based catalysts by doping other metals at single atom level is an effective way to improve their hydrogenation performance of biomass-derived molecules. However, the application of Cu-based single atom alloy catalyst in the catalytic hydrogen transfer reaction system remains to be unrevealed. Here, we develop Al2O3 supported CuPd alloy catalysts, among which the single atom alloy catalyst with Pd dispersed in single atomic level on the surface was successfully obtained by controlling the metal amount, such as Cu8Pd1/Al2O3 with the Cu/Pd Molar ratio of 8: 1. The catalytic performance for furfural hydrodeoxygenation to 2-methylfuran of the CuPd/Al2O3 catalysts, using isopropanol as a hydrogen donor, shows a volcano-type relationship to the Cu/Pd ratio. The highest 2-methylfuran yield (81% at 220 °C) was achieved on the single atom alloy catalyst Cu8Pd1/Al2O3. The underlying reaction mechanism of furfural hydrodeoxidation on Cu8Pd1/Al2O3 was revealed by a series of isotopic labeling experiments, that the furfural was firstly hydrogenated to be furfuralcohol by a metal site-mediated catalytic transfer hydrogenation pathway, and then the furfuralcohol was converted to be 2-methylfuran by a Lewis acid-mediated furan ring activation route. The weak Lewis acid site that formed by single atom Pd alloyed with CuO matrix on Cu8Pd1/Al2O3 was proposed as the active site for the highly selective generation of 2-methylfuran.
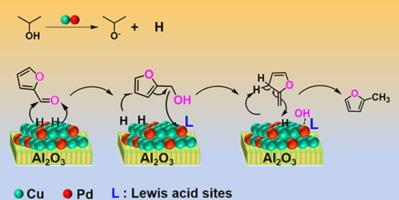
CuPd/Al2O3单原子合金催化剂有利于糠醛催化加氢脱氧生成2-甲基呋喃
在单原子水平上掺杂其他金属合金化cu基催化剂是提高其生物质衍生分子加氢性能的有效途径。然而,铜基单原子合金催化剂在催化氢转移反应体系中的应用还有待进一步研究。本研究开发了Al2O3负载型CuPd合金催化剂,其中通过控制金属量成功获得了Pd在表面单原子水平分散的单原子合金催化剂,如Cu/Pd摩尔比为8:1的Cu8Pd1/Al2O3。以异丙醇为氢供体的CuPd/Al2O3催化剂对糠醛加氢脱氧制2-甲基呋喃的催化性能与Cu/Pd比呈火山型关系。在单原子合金催化剂Cu8Pd1/Al2O3上,2-甲基呋喃的产率最高,在220 ℃时达到81%。通过一系列同位素标记实验揭示了糠醛在Cu8Pd1/Al2O3上加氢脱氧的潜在反应机理,即糠醛首先通过金属位点介导的催化转移加氢途径加氢生成糠醇,然后通过Lewis酸介导的呋喃环活化途径将糠醇转化为2-甲基呋喃。提出了单原子Pd与CuO基体在Cu8Pd1/Al2O3上合金形成的弱Lewis酸位点是2-甲基呋喃高选择性生成的活性位点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


