Study on the mechanism of ammonium carbamate in promoting the separation of chalcopyrite and arsenopyrite in oxidation systems
IF 6.3
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Arsenopyrite frequently occurs alongside chalcopyrite and is characterized by its high arsenic content. The similar surface properties of arsenopyrite and chalcopyrite pose significant challenges to their separation via flotation. This study investigated the inhibitory effect of sodium hypochlorite (NaClO) on arsenopyrite and the activation mechanism of ammonium carbamate on chalcopyrite through a series of single-mineral and artificial mixed-mineral flotation experiments. Techniques employed include atomic force microscopy (AFM), scanning electron microscopy (SEM) with energy-dispersive X-ray spectroscopy (EDS), X-ray photoelectron spectroscopy (XPS), Zeta potential analysis, and adsorption experiments. The results from flotation indicated that NaClO oxidation significantly suppressed the arsenopyrite recovery to as low as 7.80%, while chalcopyrite recovery was reduced to 54.02%. Upon the addition of ammonium carbamate, the flotation recovery of chalcopyrite increased to 81.71%, while the recovery of arsenopyrite remained largely unaffected. Further analysis with AFM, SEM, EDS, Zeta potential, adsorption tests, and XPS revealed that NaClO facilitated the formation of a hydrophilic film on the surface of arsenopyrite, reducing the adsorption of the trap on the minerals, and suppressing the hydrophobicity of arsenopyrite. Conversely, ammonium carbamate did not activate arsenopyrite but enhanced the adsorption of butyl xanthate on chalcopyrite. The results indicated that the combination of NaClO and ammonium carbamate presents an effective method for the selective separation of arsenopyrite and chalcopyrite.
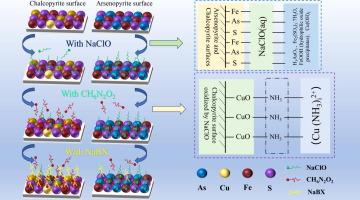
氨基甲酸铵促进氧化体系中黄铜矿和砷黄铁矿分离的机理研究
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Applied Surface Science
工程技术-材料科学:膜
CiteScore
12.50
自引率
7.50%
发文量
3393
审稿时长
67 days
期刊介绍:
Applied Surface Science covers topics contributing to a better understanding of surfaces, interfaces, nanostructures and their applications. The journal is concerned with scientific research on the atomic and molecular level of material properties determined with specific surface analytical techniques and/or computational methods, as well as the processing of such structures.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


