Photocatalytic production of H2O2 over rutile TiO2 supported with Pd nanoparticles
IF 6.3
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Hydrogen peroxide is a valuable chemical, which is commonly employed in environmental application for advanced oxidation processes and in energy application for fuel cells. Local production of H2O2 directly at sites of its consumption can eliminate risks and costs associated with transportation of concentrated H2O2 solutions. In this study, we investigated the ability of rutile or anatase TiO2 modified with Pd nanoparticles to produce hydrogen peroxide via photocatalytic oxygen reduction. Surface modification of TiO2 with Pd cocatalyst allowed H2O2 generation in both deionized water and water-methanol solution under UV-LED irradiation. Rutile TiO2 supported with Pd nanoparticles exhibited boosted photocatalytic activity in H2O2 formation compared to anatase-based photocatalyst. H2O2 was shown to predominantly form through the pathway of oxygen reduction, and observed difference in activity was attributed to different positions of energy bands in rutile and anatase phases. Methanol as an efficient electron donor substantially increased the amount of evolved H2O2. Rate constants of H2O2 generation over Pd-decorated rutile TiO2 were 1.7 μmol min−1 in deionized water and 7.4 μmol min−1 in water-methanol solution (10 vol%). This study reveals the potential of rutile for design of efficient heterostructured composites with other narrow-band semiconductors for photocatalytic generation of H2O2 under solar light.

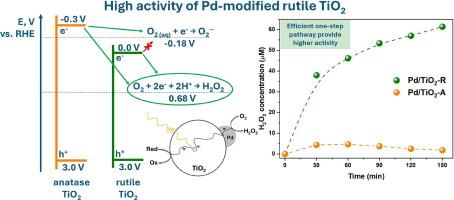
钯纳米粒子负载金红石TiO2光催化制备H2O2
过氧化氢是一种有价值的化学物质,通常用于高级氧化过程的环境应用和燃料电池的能源应用。直接在消费地点就地生产H2O2可以消除与运输浓缩H2O2溶液相关的风险和成本。在这项研究中,我们研究了金红石或锐钛矿TiO2被Pd纳米粒子修饰后,通过光催化氧还原产生过氧化氢的能力。在UV-LED照射下,用Pd助催化剂对TiO2进行表面改性,使其在去离子水和水-甲醇溶液中均能生成H2O2。与锐钛矿型光催化剂相比,Pd纳米颗粒负载的金红石型TiO2在H2O2形成过程中表现出更强的光催化活性。H2O2主要通过氧还原途径形成,活性的差异归因于金红石和锐钛矿相能带的不同位置。甲醇作为一个有效的电子供体,大大增加了H2O2的析出量。pd修饰的金红石型TiO2在去离子水中生成H2O2的速率常数为1.7 μmol min−1,在水-甲醇溶液中生成H2O2的速率常数为7.4 μmol min−1(10 vol%)。该研究揭示了金红石与其他窄带半导体设计用于太阳能光催化生成H2O2的高效异质结构复合材料的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Applied Surface Science
工程技术-材料科学:膜
CiteScore
12.50
自引率
7.50%
发文量
3393
审稿时长
67 days
期刊介绍:
Applied Surface Science covers topics contributing to a better understanding of surfaces, interfaces, nanostructures and their applications. The journal is concerned with scientific research on the atomic and molecular level of material properties determined with specific surface analytical techniques and/or computational methods, as well as the processing of such structures.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


