Flotation separation of galena and chalcopyrite by using hydroxyl radicals from an Fe2+/NaClO system as depressants
IF 6.3
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The flotation separation of galena (PbS) and chalcopyrite (CuFeS2) is not yet fully resolved due to the lack of efficient depressants. This study proposed a new depressant, i.e. an Fe2+/NaClO system, as an efficient PbS depressant. The inhibition effect of the Fe2+/NaClO system is attributed to the oxidation effect of •OH radicals from the reaction between HClO and Fe2+. The recovery of PbS is only 9.48 % and that of CuFeS2 is 82.33 % under the following conditions: pH 3.5, 4 min reaction time, 10:1 Fe2+:NaClO molar ratio and 1 × 10−2 mol/L NaClO concentration. Contact angle test results suggested that the hydrophobicity of PbS was decreased by conditioning with the Fe2+/NaClO system. X-ray photoelectron spectroscopy results showed that the S2− species on the PbS surface was oxidised into SO42−, and thus, PbSO4 occurred on the PbS surface. Furthermore, SO42− concentration reached 61.67 % of the total S species. Oxidative production coated the PbS surface well, depressing PbS flotation. However, the Fe2+/NaClO system barely changed the components of the CuFeS2 surface. The CuFeS2 surface still exhibits a high hydrophobic level. Therefore, PbS can be efficiently separated from CuFeS2 by using the Fe2+/NaClO system.
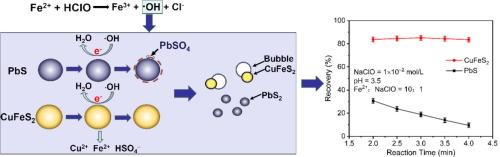
利用 Fe2+/NaClO 系统中的羟基自由基作为抑制剂浮选分离方铅矿和黄铜矿
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Applied Surface Science
工程技术-材料科学:膜
CiteScore
12.50
自引率
7.50%
发文量
3393
审稿时长
67 days
期刊介绍:
Applied Surface Science covers topics contributing to a better understanding of surfaces, interfaces, nanostructures and their applications. The journal is concerned with scientific research on the atomic and molecular level of material properties determined with specific surface analytical techniques and/or computational methods, as well as the processing of such structures.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


