Spatiotemporal Mapping of Lymphatic Metastases in Gastric Cancer Using Tumor-Trackable and Enzyme-Activatable Near-Infrared Fluorescent Nanoprobes
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Sentinel lymph node biopsy holds significant importance in cancer management, yet the challenge persists in early detection and precise resection of metastasis lymph nodes (LNs) due to the absence of specific and sensitive optical probes. This study reports metastatic LN reporters (MLRs) with an activatable optical output for accurate spatiotemporal mapping of lymphatic metastases in gastric cancer. MLRs are self-assembled entities incorporating mixed amphiphiles with a lipophilic tail and a tumor-targeting ligand or a fluorescent moiety that is caged with a switch cleavable by tumor-specific β-galactosidase (β-Gal). After draining into LNs, MLRs selectively activate their near-infrared fluorescence in the presence of spreading tumor cells. In orthotopic gastric cancer mouse models, the representative reporter MLR1 distinguishes macro/micrometastatic LNs from benign LNs and enables early detection of skip LNs metastasis patterns in a spatial-dependent manner. Such an active sensing mechanism provides a high level of sensitivity and specificity comparable to those of flow cytometry analysis. In surgically resected patient specimens, MLR1 differentiates cancerous tissues and metastatic LNs from normal tissues and benign LNs within 1 h. This study thus presents NIRF nanoprobes that permit facile detection of LN metastases in GC patient samples and highlights a generic translatable nanoprobe design for understanding metastatic progression.
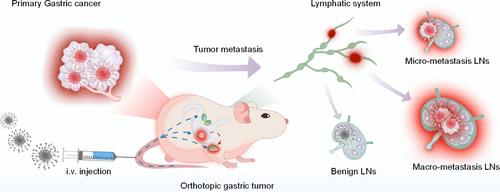
利用肿瘤可追踪和酶激活的近红外荧光纳米探针绘制胃癌淋巴转移的时空分布图
前哨淋巴结活检在癌症治疗中具有重要意义,但由于缺乏特异性和灵敏度高的光学探针,早期检测和精确切除转移淋巴结(LN)仍是一项挑战。本研究报告了具有可激活光学输出的转移淋巴结报告器(MLRs),用于准确绘制胃癌淋巴转移的时空图。MLRs 是一种自组装实体,其中包含亲脂性尾部和肿瘤靶向配体或荧光分子的混合双亲化合物,该配体被肿瘤特异性 β-半乳糖苷酶(β-Gal)可裂解的开关锁住。MLRs排入LN后,在有扩散的肿瘤细胞存在的情况下会选择性地激活其近红外荧光。在正位胃癌小鼠模型中,具有代表性的报告物 MLR1 能区分大/微转移 LN 和良性 LN,并能以空间依赖的方式及早检测跳过的 LN 转移模式。这种主动感应机制提供了与流式细胞术分析相当的高灵敏度和特异性。在手术切除的患者标本中,MLR1 能在 1 小时内将癌组织和转移性 LN 与正常组织和良性 LN 区分开来。因此,本研究提出的近红外荧光纳米探针能方便地检测 GC 患者标本中的 LN 转移,并强调了用于了解转移进展的通用可转化纳米探针设计。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


