A Polypeptosome Spray To Heal Antibiotic-Resistant Bacteria-Infected Wound by Photocatalysis-Induced Metabolism-Interference
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
With the booming antimicrobial drug resistance worldwide, traditional antibacterial agents (e.g., antibiotics) are usually powerless against superbug. Targeting antibacterial pathways different from traditional antibiotics could be an effective approach to treating wounds with a resistant bacterial infection. In this work, an antibacterial polymersome was developed to physically induce bacterial membrane damage and interfere with bacterial metabolism. First, we synthesized an antibacterial poly(ε-caprolactone)-block-poly(glutamic acid)-block-poly(Lys-stat-Phe) copolymer, which was then self-assembled into polypeptosome with the amplification of surface positive charges to disrupt bacterial membranes. In addition, the polypeptosome was further decorated with photocatalytic bismuth sulfide (Bi2S3) nanoparticles as a photocatalyst to interfere with reduced nicotinamide adenine dinucleotide (NADH) conversion. Specifically, near-infrared light generated free electrons from Bi2S3 nanoparticles could effectively interfere with NADH homeostasis to induce antibiotic-resistant bacteria death, as verified by transcriptome sequence analysis. Moreover, effective healing of antibiotic-resistant bacteria-infected wounds of mice was achieved with a spray of polypeptosome dispersion. Overall, we provided a fresh strategy to integrate bacterial membrane damage and metabolism interference functions within antibacterial polymersomes for healing antibiotic-resistant bacteria-infected wound.
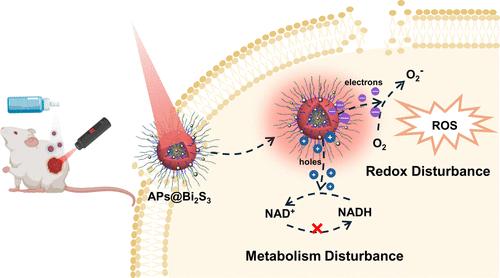
通过光催化诱导新陈代谢干扰治愈耐抗生素细菌感染伤口的多肽体喷雾剂
随着全球抗菌药物耐药性的激增,传统的抗菌剂(如抗生素)通常对超级细菌无能为力。针对不同于传统抗生素的抗菌途径可能是治疗伤口耐药菌感染的有效方法。在这项研究中,我们开发了一种抗菌聚合物组,以物理方式诱导细菌膜损伤并干扰细菌的新陈代谢。首先,我们合成了抗菌的聚(ε-己内酯)-块-聚(谷氨酸)-块-聚(Lys-stat-Phe)共聚物,然后通过放大表面正电荷自组装成多肽体,破坏细菌膜。此外,该多肽体还进一步装饰了光催化硫化铋(Bi2S3)纳米粒子,作为光催化剂干扰还原型烟酰胺腺嘌呤二核苷酸(NADH)的转化。具体而言,Bi2S3 纳米粒子产生的近红外光自由电子可有效干扰 NADH 的平衡,从而诱导抗生素耐药菌死亡,这一点已通过转录组序列分析得到验证。此外,小鼠感染抗生素细菌的伤口通过喷洒多肽体分散液得到了有效愈合。总之,我们提供了一种崭新的策略,将细菌膜损伤和代谢干扰功能整合到抗菌多聚体中,从而治愈抗生素耐药菌感染的伤口。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


