Enhanced Gut-to-Liver Oral Drug Delivery via Ligand-Modified Nanoparticles by Attenuating Protein Corona Adsorption
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The development of effective oral drug delivery systems for targeted gut-to-liver transport remains a significant challenge due to the multiple biological barriers including the harsh gastrointestinal tract (GIT) environment and the complex protein corona (PC) formation. In this study, we developed ligand-modified nanoparticles (NPs) that enable gut-to-liver drug delivery by crossing the GIT and attenuating PC formation. Specifically, mesoporous silica nanoparticles (MSNs) were functionalized with peptides targeting the neonatal Fc receptor (FcRn), capitalizing on FcRn expression in the small intestine and liver for targeted drug delivery. We showed that MSNs decorated with a small cyclic FcRn binding peptide (MSNs-FcBP) obtained enhanced diffusion in intestinal mucus and superior transportation across the intestine compared to unmodified MSNs and MSNs decorated with a large IgG Fc fragment (MSNs-Fc), which correlated with diminished protein adsorption and weaker interaction with mucin. After entering the blood circulation, reduced serum PC formation by MSNs-FcBP reduces the proteolytic and phagocytic propensity of the reticuloendothelial system, ultimately ameliorating accumulation in hepatocytes. Pharmacokinetic and pharmacodynamic studies in diabetic mice revealed that MSNs-FcBP effectively transported the therapeutic agent exenatide across the intestinal epithelium, leading to a significant hypoglycemic response and improved glucose tolerance. This study underscores the critical role of ligand selection in limiting protein corona formation, thereby significantly enhancing gut-to-liver drug delivery by increasing mucus permeation and minimizing serum–protein interactions. The effective delivery of exenatide in diabetic mice illustrates the potential of this strategy to optimize oral drug bioavailability and therapeutic efficacy.
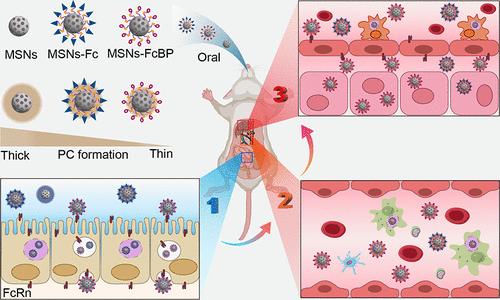
由于存在多种生物障碍,包括严酷的胃肠道(GIT)环境和复杂的蛋白电晕(PC)形成,因此开发有效的口服药物从肠道到肝脏的靶向传输系统仍然是一项重大挑战。在这项研究中,我们开发了配体修饰的纳米颗粒(NPs),通过穿越胃肠道和减弱 PC 的形成,实现从肠道到肝脏的药物输送。具体来说,介孔二氧化硅纳米颗粒(MSNs)用靶向新生儿 Fc 受体(FcRn)的肽进行了功能化,利用小肠和肝脏中 FcRn 的表达进行靶向给药。我们的研究表明,与未修饰的 MSNs 和修饰了大分子 IgG Fc 片段的 MSNs(MSNs-Fc)相比,修饰了小分子环状 FcRn 结合肽(MSNs-FcBP)的 MSNs 在肠粘液中的扩散能力更强,在肠道中的运输效果更好,这与蛋白质吸附力减弱和与粘蛋白的相互作用减弱有关。进入血液循环后,MSNs-FcBP 形成的血清 PC 减少,从而降低了网状内皮系统的蛋白水解和吞噬倾向,最终改善了肝细胞中的积聚。对糖尿病小鼠进行的药代动力学和药效学研究表明,MSNs-FcBP 能有效地将治疗药物艾塞那肽通过肠上皮细胞转运,从而产生明显的降糖反应并改善葡萄糖耐量。这项研究强调了配体选择在限制蛋白电晕形成方面的关键作用,从而通过增加粘液渗透性和最大限度地减少血清蛋白相互作用,大大提高了从肠道到肝脏的药物输送。在糖尿病小鼠体内有效输送艾塞那肽说明了这种策略在优化口服药物生物利用度和疗效方面的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


