Electrochemical Synthesis of an N-Arylpyridazinone: Discovery and Scale-Up
IF 3.1
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
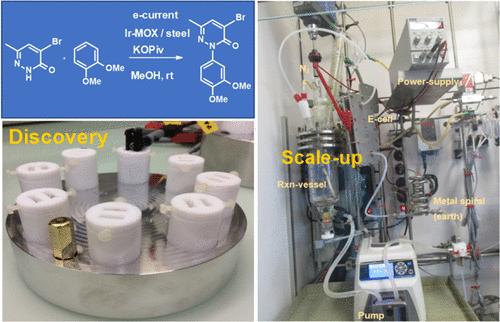
An electrochemical NH/CH-coupling of a functionalized pyridazinone with veratrole was developed to create the central C–N bond of an agrochemical intermediate. Electrolysis was performed in methanol using potassium pivalate, which served a dual role as both a mild catalytic base and a supporting electrolyte; H2 is generated as the sole stoichiometric byproduct. Mechanistic studies suggest the formation of a N-centered radical on the pyridazinone nitrogen that adds to the veratrole pi-system; closure of the catalytic cycle involves further one-electron oxidation and deprotonation. Initial optimization in a batch electrochemical cell was followed by optimization of a loop-flow process that provided significant yield improvements from batch. The loop-flow electrochemical process was successfully scaled to a reaction volume of 2 L.
一种 N-芳基哒嗪酮的电化学合成:发现和推广
我们开发了一种电化学 NH/CH 偶联方法,将官能化的哒嗪酮与 veratrole 偶联,从而生成一种农用化学品中间体的中心 C-N 键。电解是在甲醇中使用特戊酸钾进行的,特戊酸钾具有温和催化基和支撑电解质的双重作用;生成的 H2 是唯一的化学副产物。机理研究表明,哒嗪酮氮上形成了一个 N-中心自由基,该自由基加入到veratrole pi-系统中;催化循环的结束涉及进一步的单电子氧化和去质子化。在间歇式电化学电池中进行初步优化后,又对环流工艺进行了优化,与间歇式工艺相比,该工艺的产量有了显著提高。环流电化学工艺已成功扩展到 2 升的反应体积。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


