Metal-Free Synthesis of 4-Bromoisoquinolines through Brominative Annulation of 2-Alkynyl Arylimidate Using In Situ-Generated Transient Bromoiodane
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Herein, we report the in situ-generated transient bromoiodane-mediated brominative annulation of 2-alkynyl arylimidate for the synthesis of 4-bromoisoquinolines at room temperature. Using a simple hypervalent iodine reagent PIDA as a mild oxidant and potassium bromide as the halogen source, a broad range of valuable 4-bromoisoquinolines can be synthesized in excellent yields. The reaction features readily available chemicals, mild metal-free conditions, and high functional group tolerance, providing an efficient alternative for the construction of halogenated isoquinolines.
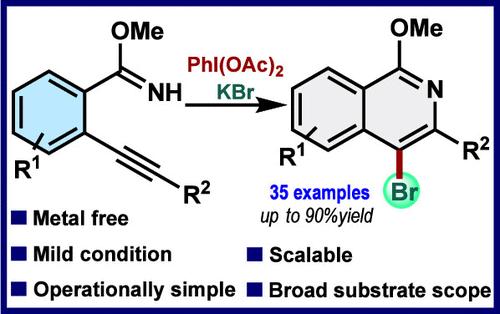
在此,我们报告了由原位生成的瞬时溴碘烷介导的 2-炔基芳基亚胺的溴化环化反应,从而在室温下合成 4-溴异喹啉类化合物。使用简单的高价碘试剂 PIDA 作为温和的氧化剂,并使用溴化钾作为卤素源,就能以极高的产率合成出多种有价值的 4-溴异喹啉类化合物。该反应具有化学品易得、条件温和无金属、官能团耐受性高等特点,为构建卤代异喹啉提供了一种高效的替代方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


